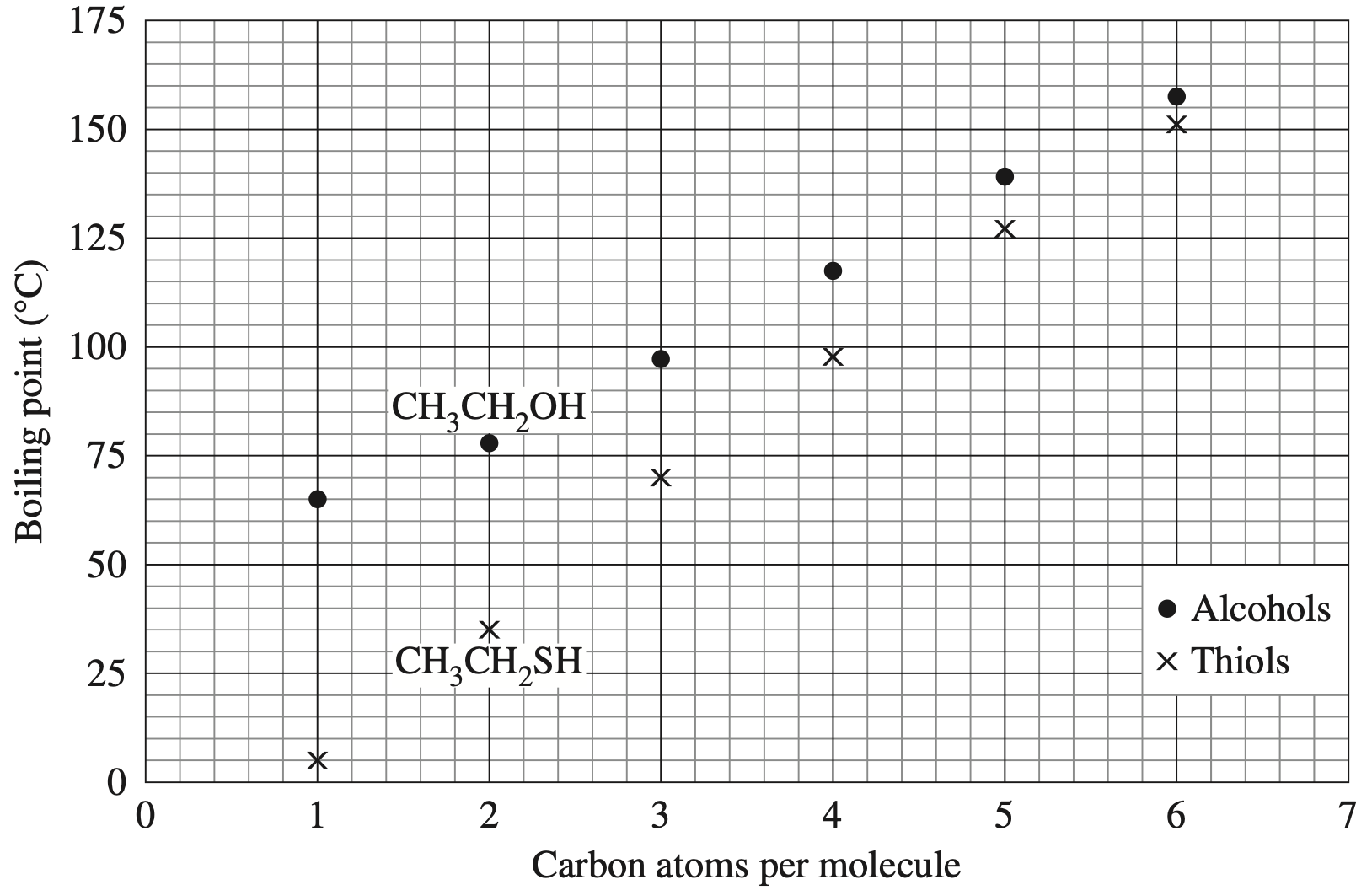
Which of the following correctly lists the compounds in order of increasing boiling point?
- Heptane < heptan-2-one < heptan-1-o1 < heptanoic acid
- Heptane < heptan-1-o1 < heptan-2-one < heptanoic acid
- Heptanoic acid < heptan-2-one < heptan-1-o1 < heptane
- Heptanoic acid < heptan-1-o1 < heptan-2-one < heptane