How many structural isomers have the molecular formula \(\ce{C3H6BrCl}\)?
- 4
- 5
- 6
- 7
Aussie Maths & Science Teachers: Save your time with SmarterEd
How many structural isomers have the molecular formula \(\ce{C3H6BrCl}\)?
\(B\)
Isomers are:
\(\Rightarrow B\)
How many structural isomers have the molecular formula \( \ce{C3H6F2} \)?
\(C\)
\(\Rightarrow C\)
Some isomers with the formula \( \ce{C4H8O} \) are shown.
Name ONE pair of functional group isomers and ONE pair of chain isomers from the structures above. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
Functional Group: Butan-2-one and butanal
Functional Group: Butan-2-one and butanal
Draw the structural formulae and name all possible isomers of hexane. (3 marks)
--- 7 WORK AREA LINES (style=lined) ---
How many isomers are there of \(\ce{C3H6ClF}\)?
`C`
`=>C`
--- 0 WORK AREA LINES (style=blank) ---
--- 4 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. Successful answers should have one of the following:
b. Functional Group isomers
c. Tollens’ Test:
a. Successful answers should have one of the following:
b. Functional Group isomers
c. Tollens’ Test:
The structures of four isomers are shown.
Which statement is correct?
`C`
Consider each option:
`=> C`
A bottle labelled 'propanol' contains one of two isomers of propanol.
--- 6 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. Isomer 1:
Isomer 2:
b. Identifying isomers with \( \ce{^13C NMR} \) spectroscopy:
c.
a. Isomer 1:
Isomer 2:
b. Identifying isomers with \( \ce{^13C NMR} \) spectroscopy:
c.
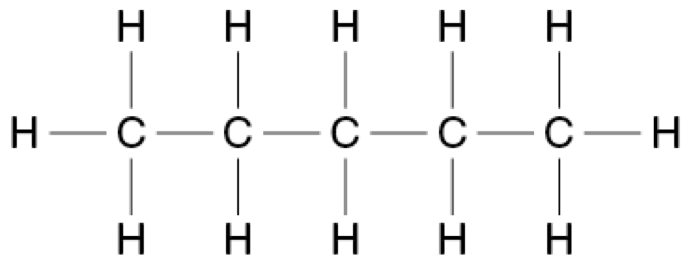
A straight-chained alkane has a molar mass of 72.146 g mol ¯1.
Provide the structural formulae for this alkane and all of its isomers.
Name these molecules using IUPAC conventions. (4 marks)
--- 8 WORK AREA LINES (style=lined) ---
Straight chained alkane (Pentane):
2-methylbutane:
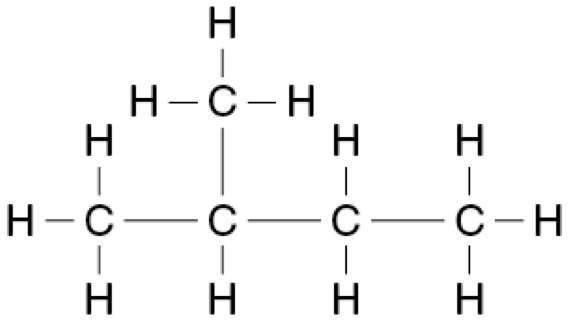
2,2-dimethylpropane:

Straight chained alkane (Pentane):

2-methylbutane:

2,2-dimethylpropane:
