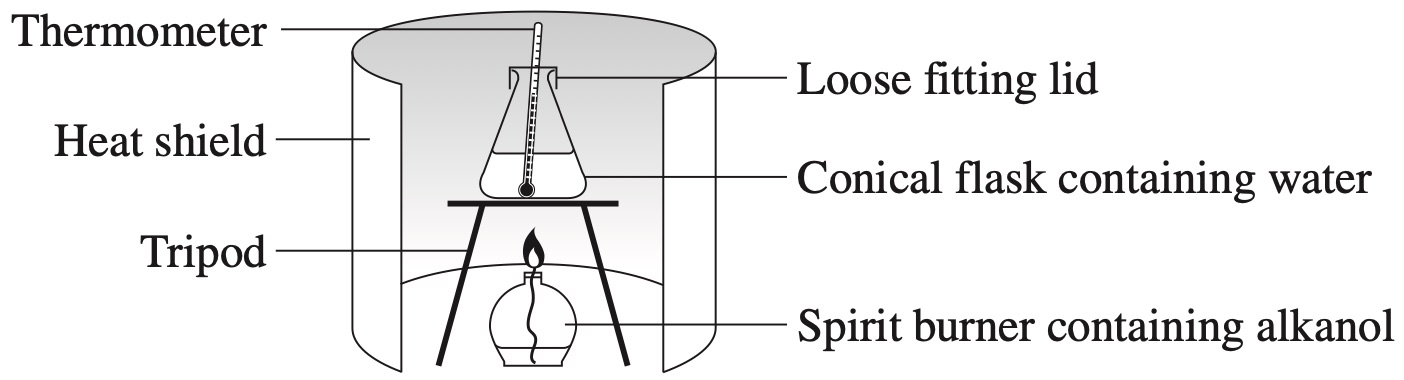
A fuel has these enthalpies of combustion: –2057.8 kJ mol\(^{-1}\) and –48.9 kJ g\(^{-1}\).
Which of the following correctly identifies the fuel?
- Ethanol \(\left(M M=46.1 \text{ g mol}^{-1}\right)\)
- Propane \(\left(M M=44.1 \text{ g mol}^{-1}\right)\)
- Propene \(\left(M M=42.1 \text{ g mol}^{-1}\right)\)
- Hydrogen \(\left(M M=2.02 \text{ g mol}^{-1}\right)\)