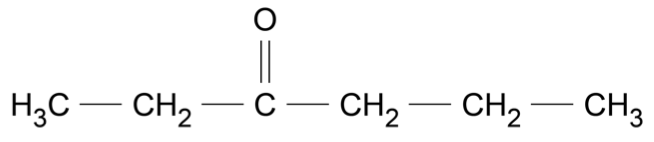Compounds \(\text{A}\) and \(\text{B}\) are isomers with formula \(\ce{C3H7X}\), where \(\ce{X}\) is a halogen. The mass spectrum for compound \(\text{A}\) is shown. Compounds \(\text{A}\) and \(\text{B}\) undergo substitution reactions in the presence of hydroxide ions, producing alcohols \(\text{C}\) and \(\text{D}\). Compound \(\text{D}\) can be oxidised to a ketone; compound \(\text{C}\) can also be oxidised, but does not produce a ketone. Compound \(\text{E}\) can be produced by refluxing 3-methylbutanoic acid, with one of the alcohols \(\text{C}\) or \(\text{D}\), in the presence of a catalyst. The \({ }^1 \text{H NMR}\) spectrum for compound \(\text{E}\) contains the following features. Draw the structure of compounds \(\text{A}\), \(\text{B}\) and \(\text{E}\). Explain your answer with reference to the information provided. (7 marks) --- 20 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2022 VCE 5*
A chemist uses spectroscopy to identify an unknown organic molecule, Molecule \(\text{J}\), that contains chlorine. The \({}^{13}\text{C NMR}\) spectrum of Molecule \(\text{J}\) is shown below. The infra-red (IR) spectrum of Molecule \(\text{J}\) is shown below. --- 2 WORK AREA LINES (style=lined) --- The mass spectrum of Molecule \(\text{J}\) is shown below --- 2 WORK AREA LINES (style=lined) --- The \({ }^1 \text{H NMR}\) spectrum of Molecule \(\text{J}\) is shown below. --- 3 WORK AREA LINES (style=lined) --- --- 5 WORK AREA LINES (style=blank) ---
CHEMISTRY, M8 2021 VCE 7*
Two students are given a homework assignment that involves analysing a set of spectra and identifying an unknown compound. The unknown compound is one of the molecules shown below. The \(^{13}\text{C NMR}\) spectrum of the unknown compound is shown below. --- 1 WORK AREA LINES (style=lined) --- --- 6 WORK AREA LINES (style=lined) --- --- 1 WORK AREA LINES (style=lined) --- --- 4 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2021 VCE 11 MC
The spectroscopy information for an organic molecule is given below.
| number of peaks in \({}^{13}\text{C NMR}\) | 2 |
| number of sets of peaks in \({}^{1}\text{H NMR}\) | 3 |
| m/z of the last peak in the mass spectrum | 60 |
| infra-red (IR) spectrum | an absorption peak appears at 3350 cm\(^{-1}\) |
The organic molecule is
CHEMISTRY, M8 2023 VCE 7-1*
Molecule \(\text{V}\) contains only carbon atoms, hydrogen atoms and one oxygen atom. The mass spectrum of molecule \(\text{V}\) is shown below. --- 4 WORK AREA LINES (style=lined) --- --- 1 WORK AREA LINES (style=lined) --- The \({ }^1 \text{H NMR}\) spectrum of molecule \(\text{V}\) is shown below. --- 2 WORK AREA LINES (style=lined) --- The \({ }^{13} \text{C NMR}\) spectrum of molecule \(\text{V}\) is shown below. --- 8 WORK AREA LINES (style=blank) ---
CHEMISTRY, M8 2023 HSC 36
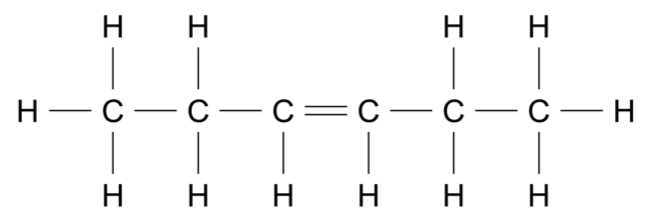
An organic reaction pathway involving compounds \(\text{A, B,}\) and \(\text{C}\) is shown in the flow chart.
The molar mass of \(\text{A}\) is 84.156 g mol\(^{-1}\).
A chemist obtained some spectral data for the compounds as shown.
| \( \text{Data from} \ ^{1} \text{H NMR spectrum of compound C} \) | ||
| \( Chemical \ Shift \ \text{(ppm)} \) | \( Relative \ peak \ area \) | \( Splitting \ pattern \) |
| \(1.01\) | \(3\) | \(\text{Triplet}\) |
| \(1.05\) | \(3\) | \(\text{Triplet}\) |
| \(1.65\) | \(2\) | \(\text{Multiplet}\) |
| \(2.42\) | \(2\) | \(\text{Triplet}\) |
| \(2.46\) | \(2\) | \(\text{Quartet}\) |
| \( ^{1} \text{H NMR chemical shift data}\) | |
| \( Type \ of \ proton \) | \( \text{δ/ppm} \) |
| \( \ce{R - C\textbf{H}3,R - C\textbf{H}2 - R}\) | \(0.7-1.7\) |
| \( \left.\begin{array}{l}\ce{\textbf{H}3C - CO - \\-C\textbf{H}2 - CO -}\end{array}\right\} \begin{aligned} & \text { (aldehydes, ketones,} \\ &\text{carboxylic acids or esters) }\end{aligned}\) | \(2.0-2.6\) |
| \( \ce{R - C\textbf{H}O} \) | \(9.4-10.00\) |
| \( \ce{R - COO\textbf{H}} \) | \(9.0-13.0\) |
Identify the functional group present in each of compounds \(\text{A}\) to \(\text{C}\) and draw the structure of each compound. Justify your answer with reference to the information provided. (9 marks)
--- 28 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2019 HSC 26b
Explain why a chemist should use more than one spectroscopic technique to identify an organic compound. Use TWO spectroscopic techniques to support your answer. (3 marks)
CHEMISTRY, M8 2019 HSC 26a
The following data were obtained for an organic compound containing carbon, hydrogen and oxygen. The compound is a colourless liquid that reacts with sodium carbonate powder to produce bubbles.
What is the structural formula of this compound? Justify your answer with reference to the information given on its reactivity and to at least THREE of the provided spectra. (5 marks)
CHEMISTRY, M8 2020 HSC 30
A chemist discovered a bottle simply labelled \( '\ce{C5H10O2}' \).
To confirm the molecular structure of the contents of the bottle, a sample was submitted for analysis by infrared spectroscopy and \( \ce{^1H} \) and \( \ce{^13C NMR} \) spectroscopy. The resulting spectra are shown.
Draw a structural formula for the unknown compound that is consistent with all of the information provided. Justify your answer with reference to the information provided. (7 marks)
--- 20 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2022 HSC 30
The following spectra were obtained for an unknown organic compound.
In the space provided, draw and name the unknown compound that is consistent with all the information provided. Justify your answer with reference to the information provided. (7 marks)
--- 15 WORK AREA LINES (style=lined) ---