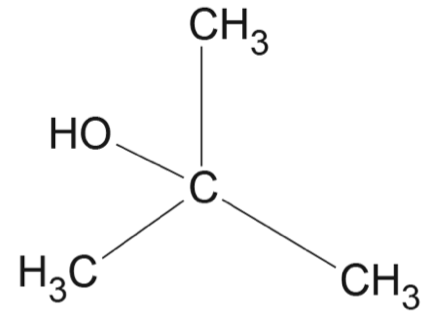
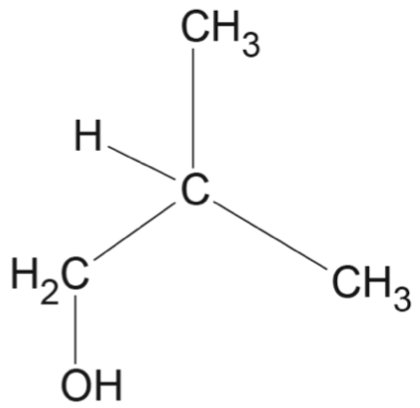
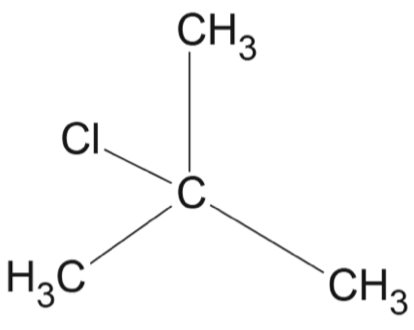
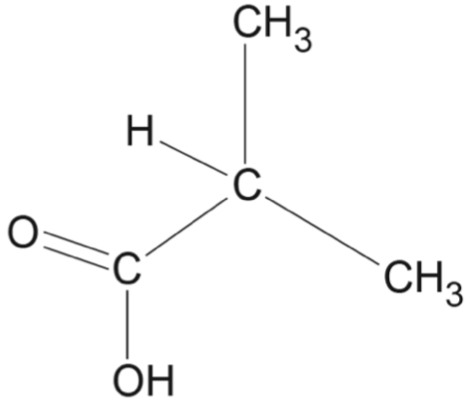
Which row of the table correctly matches the reaction type with the reactant(s), catalyst and product(s)?
CHEMISTRY, M7 2019 HSC 34
CHEMISTRY, M7 2020 HSC 29
| The flow chart shows reactions involving five different organic compounds, | to |
| Draw the structure of each compound | to | in the corresponding space provided. (5 marks) |
--- 0 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2022 HSC 33
Analyse how a student could design a chemical synthesis process to be undertaken in the school laboratory. In your response, use a specific process relating to the synthesis of an organic compound, including a chemical equation, and refer to:
- selection of reagent(s)
- reaction conditions
- any potential hazards and any safety precautions to minimise the risk
- yield and purity of the product(s). (8 marks)
--- 25 WORK AREA LINES (style=lined) ---
CHEMISTRY, M7 2022 HSC 11 MC
Cyclohexanol is an alcohol and undergoes a dehydration reaction with sulfuric acid as shown.
What is the major organic product of this reaction?
CHEMISTRY, M7 2021 HSC 26
A sequence of chemical reactions, starting with 2-methylprop-1-ene, is shown in the flow chart.
CHEMISTRY, M7 2021 HSC 13 MC
CHEMISTRY, M7 2021 HSC 7 MC
Methanol undergoes a substitution reaction using hydrogen bromide.
Compared to methanol, the product of this reaction has a
- lower boiling point.
- lower molecular mass.
- greater solubility in water.
- different molecular geometry at the carbon atom.