Below is a reaction pathway beginning with hex-3-ene.
- Write the IUPAC name of Compound J in the box provided. (1 mark)
--- 0 WORK AREA LINES (style=lined) ---
- State the reagent(s) required to convert hex-3-ene to hexan-3-ol in the box provided. (1 mark)
--- 0 WORK AREA LINES (style=lined) ---
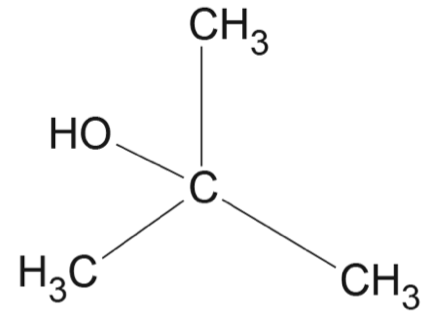
- Draw the structural formula for a tertiary alcohol that is an isomer of hexan-3-ol. (1 mark)
--- 6 WORK AREA LINES (style=lined) ---
- Hexan-3-ol is reacted with Compound M under acidic conditions to produce Compound L.
- Draw the semi-structural formula for Compound M in the box provided on the image above. (1 mark)
--- 0 WORK AREA LINES (style=lined) ---
- i. Draw the semi-structural formula for Compound K in the box provided on the image above. (1 mark)
--- 0 WORK AREA LINES (style=lined) ---
- ii. Name the class of organic compound (homologous series) to which Compound K belongs. (1 mark)
--- 1 WORK AREA LINES (style=lined) ---
- What type of reaction produces Compound K from hexan-3-ol? (1 mark)
--- 1 WORK AREA LINES (style=lined) ---