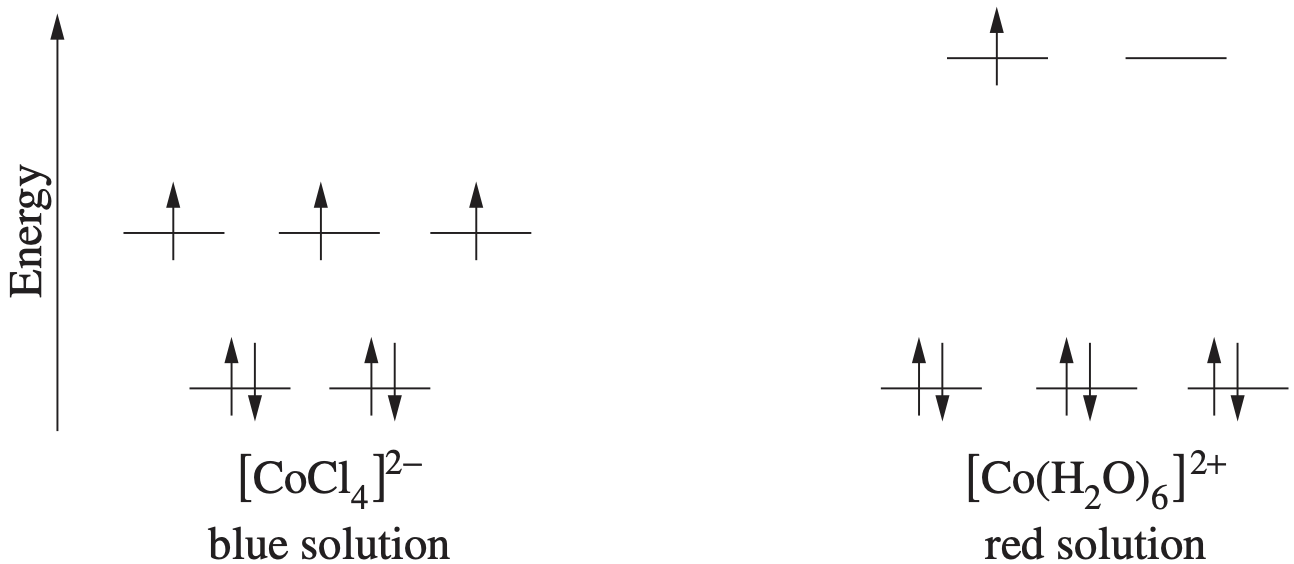
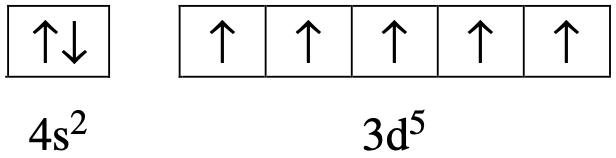
Which of the alternatives below identifies the electron configuration of the cation and anion present in the compound magnesium oxide?
\begin{align*}
\begin{array}{l}
\rule{0pt}{2.5ex} \ \rule[-1ex]{0pt}{0pt}& \\
\rule{0pt}{2.5ex}\textbf{A.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{B.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{C.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{D.}\rule[-1ex]{0pt}{0pt}\\
\end{array}
\begin{array}{|c|c|}
\hline
\rule{0pt}{2.5ex}\text{Cation}\rule[-1ex]{0pt}{0pt}& \text{Anion} \\
\hline
\rule{0pt}{2.5ex} 1s^2\,2s^2\,2p^6\,3s^1\rule[-1ex]{0pt}{0pt}& 1s^2\,2s^2\,2p^6\\
\hline
\rule{0pt}{2.5ex} 1s^2\,2s^2\,2p^6\rule[-1ex]{0pt}{0pt}& 1s^2\,2s^2\,2p^6\\
\hline
\rule{0pt}{2.5ex} 1s^2\,2s^2\,2p^6\,3s^2\rule[-1ex]{0pt}{0pt}& 1s^2\,2s^2\,2p^4 \\
\hline
\rule{0pt}{2.5ex} 1s^2\,2s^2\,2p^6\rule[-1ex]{0pt}{0pt}& 1s^2\,2s^2\,2p^6\,3s^2\,3p^6 \\
\hline
\end{array}
\end{align*}