An indicator solution was obtained by boiling a flower in water.
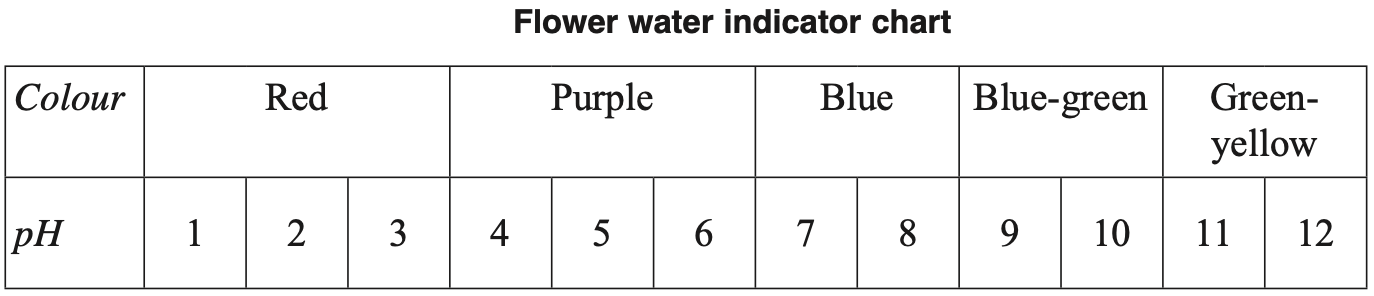
Two solutions were tested with this indicator.
Which row of the table correctly identifies the colour of each solution?
| \( \ce{H2SO4} \ (1 \ × \ 10^{-5} \ \text{mol L}^{-1}) \) | \( \ce{NaOH} \ (5 \ × \ 10^{-5} \ \text{mol L}^{-1}) \) | |
| \(\text{A.}\) | \( \text{Red} \) | \( \text{Green-yellow} \) |
| \(\text{B.}\) | \( \text{Red} \) | \( \text{Blue-green} \) |
| \(\text{C.}\) | \( \text{Purple} \) | \( \text{Blue-green} \) |
| \(\text{D.}\) | \( \text{Purple} \) | \( \text{Green-yellow} \) |