The diagram shows a plant cell organelle.
Name the organelle shown. (1 mark)
--- 1 WORK AREA LINES (style=lined) ---
Aussie Maths & Science Teachers: Save your time with SmarterEd
The graph shows the relationship between substrate concentration and reaction rate in an enzyme-catalysed reaction.
Why does the reaction rate NOT continue to increase after point \(X\)?
\(D\)
→ The reason substrate concentration does not increase but reaction rate is still at it’s peak is due to all the enzyme active sites being occupied.
\(\Rightarrow D\)
--- 4 WORK AREA LINES (style=lined) --- --- 3 WORK AREA LINES (style=lined) --- i. Studying the process of photosynthesis: → Leads to an understanding of how oxygen is produced. → Leads to an understanding of how raw materials can be produced for a range of human needs, such as in medicinal and agricultural fields. ii. Functions of photosynthesis products: → The products of photosynthesis are oxygen and sugar. → They are used in cellular respiration in all cells which contain mitochondria, in both animals and plants. i. Studying the process of photosynthesis: → Leads to an understanding of how oxygen is produced. → Leads to an understanding of how raw materials can be produced for a range of human needs, such as in medicinal and agricultural fields. ii. Functions of photosynthesis products: → The products of photosynthesis are oxygen and sugar. → They are used in cellular respiration in all cells which contain mitochondria, in both animals and plants.
Analyse the impact of the development of the electron microscope on the understanding of chloroplast structure and function. (7 marks)
--- 14 WORK AREA LINES (style=lined) ---
→ Using light microscopes, scientists were able to view and identify chloroplasts. However, it wasn’t until the development of the electron microscope with its greater magnification and resolution, that scientists were able to view a chloroplast’s internal structure.
→ Structures such as the grana, stroma and thylakoids could then be identified. The role of each in the process of photosynthesis could then be studied.
→ Thylakoids are flattened, hollow discs which are arranged in stacks called grana. The stacking of the layers into grana increases stability and surface area for the capture of light.
→ The membranes of these thylakoids contain chlorophyll and are the site for the light-dependent reactions of photosynthesis.
→ The space outside the thylakoid is called the stroma, which is an aqueous fluid present within the inner membrane of the chloroplast. It contains DNA, ribosomes, lipid droplets and starch granules. This is where the light independent reactions, the Calvin cycle, takes place.
→ The functions described would not have been linked to the internal structures of the chloroplast without the development of an electron microscope.
→ Using light microscopes, scientists were able to view and identify chloroplasts. However, it wasn’t until the development of the electron microscope with its greater magnification and resolution, that scientists were able to view a chloroplast’s internal structure.
→ Structures such as the grana, stroma and thylakoids could then be identified. The role of each in the process of photosynthesis could then be studied.
→ Thylakoids are flattened, hollow discs which are arranged in stacks called grana. The stacking of the layers into grana increases stability and surface area for the capture of light.
→ The membranes of these thylakoids contain chlorophyll and are the site for the light-dependent reactions of photosynthesis.
→ The space outside the thylakoid is called the stroma, which is an aqueous fluid present within the inner membrane of the chloroplast. It contains DNA, ribosomes, lipid droplets and starch granules. This is where the light independent reactions, the Calvin cycle, takes place.
→ The functions described would not have been linked to the internal structures of the chloroplast without the development of an electron microscope.
\(B\)
By Elimination
→ Diffusion moves molecules from high to low concentration (Eliminate A).
→ Active transport uses energy to move molecules against the concentration gradient, therefore from low to high (Eliminate C).
→ Passive transport refers to the diffusion of simple molecules which can ‘squeeze between’ the phospholipid molecules of the membrane. Sugars are too large to do this and require carrier or channel proteins. (Eliminate D).
\(\Rightarrow B\)
Construct a flow chart to summarise the main steps and products of the light-independent reactions of photosynthesis. (5 marks)


\begin{array} {|l|l|} --- 4 WORK AREA LINES (style=lined) --- a. \begin{array} {|l|l|} → Due to this, when water evaporates or transpires, other water molecules are pulled up the xylem vessels. → This is one of the core mechanisms that drives the movement of water from roots to leaves. a. \begin{array} {|l|l|} → Due to this, when water evaporates or transpires, other water molecules are pulled up the xylem vessels. → This is one of the core mechanisms that drives the movement of water from roots to leaves.
\hline
\rule{0pt}{2.5ex}\textit{Dependent variable}\rule[-1ex]{0pt}{0pt} & \text{•} \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \\
\hline
\rule{0pt}{2.5ex}\textit{Control}\rule[-1ex]{0pt}{0pt} & \text{•} \\
\hline
\rule{0pt}{2.5ex}\textit{Variables to be kept constant}\rule[-1ex]{0pt}{0pt} & \text{•} \\ & \text{•} \\
\hline
\end{array}
\hline
\rule{0pt}{2.5ex}\textit{Dependent variable}\rule[-1ex]{0pt}{0pt} & \text{• Amount of water lost} \\
\hline
\rule{0pt}{2.5ex}\textit{Control}\rule[-1ex]{0pt}{0pt} & \text{• Plant kept in the dark} \\
\hline
\rule{0pt}{2.5ex}\textit{Variables to be kept constant}\rule[-1ex]{0pt}{0pt} & \text{• Plant type} \\ & \text{• Temperature} \\
\hline
\end{array}
b. → Water molecules have chemical properties which make them cohesive.
\hline
\rule{0pt}{2.5ex}\textit{Dependent variable}\rule[-1ex]{0pt}{0pt} & \text{• Amount of water lost} \\
\hline
\rule{0pt}{2.5ex}\textit{Control}\rule[-1ex]{0pt}{0pt} & \text{• Plant kept in the dark} \\
\hline
\rule{0pt}{2.5ex}\textit{Variables to be kept constant}\rule[-1ex]{0pt}{0pt} & \text{• Plant type} \\ & \text{• Temperature} \\
\hline
\end{array}
b. → Water molecules have chemical properties which make them cohesive.
One test used for random breath testing in NSW involved crystals of potassium dichromate reacting with ethanol. In this reaction the orange dichromate ion, \(\ce{Cr2O7}^{2-}\), changes to the green chromium ion, \(\ce{Cr^3+}\).
Which statement is true for this reaction?
\(C\)
Let \(x\) equal the oxidation number of Cr in \(\ce{Cr2O7}^{2-}\).
\(2x+ 7 \times -2 = -2\)
\(2x= 12\)
\(x=6\)
The Chromium has been reduced as the oxidation number has decreased from 6 to 3, thus it gains electrons and the oxidation state is lower.
\(\Rightarrow C\)
Use the information provided to answer Questions 13 and 14.
\begin{array} {|l|}
\hline \text{This equation represents a common redox reaction.} \\ \ \ \ \ce{Cr2O7^{2-}(aq) + 14H+(aq) + 6Fe^{2+}(aq) \rightarrow 2Cr^{3+}(aq) + 6Fe^{3+}(aq) + 7H2O(l)} \\
\hline \end{array}
Question 13
What is the oxidising agent in the reaction?
Question 14
What is the value of \(\ce{E}_{\text {cell }}^{\ominus}\) for the reaction?
\(\text{Question 13:}\ D\)
\(\text{Question 14:}\ A\)
\(\text{Question 13:}\)
The oxidising agent is the chemical that undergoes reduction. The chromium changes its oxidation number from \(+6\) to \(+3\) indication reduction.
Thus it is the dichromate ion which undergoes reduction.
\(\Rightarrow D\)
\(\text{Question 14:}\)
From the list of standard potentials:
The reduction voltage of dichromate ions is 1.36 \(\text{V}\)
The oxidation of \(\ce{Fe^2+}\) ions is -0.77 \(\text{V}\)
\(1.36 + -0.77= 0.59\ \text{V}\)
\(\Rightarrow A\)
A diagram of a simple cell is shown.
Which of the following occurs when the cell is in operation?
\(B\)
→ Copper is a more active metal than silver.
→ Thus the copper electrode will be the anode and the silver electrode will be the cathode, and in REDOX reactions, electrons flow from the anode to the cathode.
→ Hence the copper electrode will lose electrons.
\(\Rightarrow B\)
What happens to \(\ce{Fe^2+} \) in the following reaction?
\( \ce{Sn^4+} + \ce{Fe^2+} \rightarrow \ce{Sn^3+} + \ce{Fe^3+} \)
\(C\)
→ The increase from \(2^+\) to \(3^+\) indicates oxidation.
→ During oxidation, the species loses electrons.
\(\Rightarrow C\)
Which of the following are the balanced products of the following reaction:
\(\ce{H3PO4 + 3NaOH \rightarrow ?}\)
\(A\)
→ An acid base reaction result in the formation of a salt (\ce{Na3PO4}) and water (\ce{H2O}\) and 3 (\ce{H2O}\) are required to balance the equation.
\(\Rightarrow A\)
Calculate the mass of solid sodium hydrogen carbonate required to make 250 mL of 0.12 mol L\(^{-1}\) solution. (2 marks)
\(2.52\ \text{g} \)
\(\ce{n(NaHCO3\ \text{in solution})\ = 0.12 \times \dfrac{250}{1000} = 0.03\ \text{mol}}\)
\(\ce{MM(NaHCO3) = 22.99 + 1.008 + 12.01 + 3 \times 16.00 = 84.0\ \text{g mol}^{-1}} \)
\(\ce{m(NaHCO3) = n \times MM = 0.03 \times 84.0 = 2.52\ \text{g}} \)
A student studying the mass change that occurs during fermentation added glucose, water and yeast to a flask and stoppered the flask with some cotton wool.
The student measured the mass of the flask daily for seven days. The table shows the data collected.
\begin{array} {|c|c|}
\hline
\rule{0pt}{2.5ex}\ \ \ \textit{Day}\ \ \ \rule[-1ex]{0pt}{0pt} & \ \ \textit{Mass}\ \text{(g)}\ \ \\
\hline
\rule{0pt}{2.5ex} 1 \rule[-1ex]{0pt}{0pt} & 381.05\\
\hline
\rule{0pt}{2.5ex} 2 \rule[-1ex]{0pt}{0pt} & 376.96\\
\hline
\rule{0pt}{2.5ex} 3 \rule[-1ex]{0pt}{0pt} & 373.42\\
\hline
\rule{0pt}{2.5ex} 4 \rule[-1ex]{0pt}{0pt} & 370.44\\
\hline
\rule{0pt}{2.5ex} 5 \rule[-1ex]{0pt}{0pt} & 370.42\\
\hline
\rule{0pt}{2.5ex} 6 \rule[-1ex]{0pt}{0pt} & 370.40\\
\hline
\rule{0pt}{2.5ex} 7 \rule[-1ex]{0pt}{0pt} & 370.39\\
\hline
\end{array}
--- 2 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. 59.37 moles
b. 5395.92 g
a. \(\ce{MM (CO2) = 12.01 + 2 \times 16.0 = 44.01 g mol^{-1}}\)
\(\ce{Mass CO2 released = 2613.08 g}\)
\[\ce{n(CO2 released) = \frac{2613.08}{44.01} = 59.37 moles}\]
b. \(\ce{C6H12O6 \rightarrow 2C2H6O + 2CO2}\)
→ The moles of \(\ce{CO2}\) released and the reaction’s molar ratio can be used to calculate the mass of glucose that underwent fermentation.
→ Molar ratio between glucose and carbon dioxide = \(1:2\)
\(\ce{n(C6H12O6) = \dfrac{1}{2} \times 59.37 = 29.685\ \text{mol}} \)
\(\ce{MM(C6H12O6) = 180.56\ \text{g mol}^{-1}} \)
\(\ce{m(C6H12O6) = 29.685 \times 180.56 = 5395.92\ \text{g}} \)
A chemist analysed aspirin tablets for quality control. The initial step of the analysis was the standardisation of a \(\ce{NaOH}\) solution. Three 25.00 mL samples of a 0.1034 mol L\(^{-1}\) solution of standardised \(\ce{HCl}\) were titrated with the \( \ce{NaOH} \) solution. The average volume required for neutralisation was 25.75 mL.
Three flasks were prepared each containing a mixture of 25 mL of water and 10 mL of ethanol. An aspirin tablet was dissolved in each flask. The aspirin in each solution was titrated with the standardised \(\ce{NaOH}\) solution according to the following equation:
\(\ce{C9H8O4(aq) + NaOH(aq) \rightarrow C9H7O4Na(aq) + H2O(l)}\)
The following titration results were obtained.
\begin{array} {|c|c|}
\hline
\rule{0pt}{2.5ex}\textit{Tablet}\rule[-1ex]{0pt}{0pt} & \textit{Volume}\ \text{(mL)}\\
\hline
\rule{0pt}{2.5ex}\text{1}\rule[-1ex]{0pt}{0pt} & 16.60\\
\hline
\rule{0pt}{2.5ex}\text{2}\rule[-1ex]{0pt}{0pt} & 16.50\\
\hline
\rule{0pt}{2.5ex}\text{3}\rule[-1ex]{0pt}{0pt} & 16.55\\
\hline
\end{array}
a. \(\ce{0.1004 mol L^{-1}}\)
b. \(\ce{299.2 mg}\)
a. \(\ce{n(HCl) = c \times V = 0.1034 \times 0.02500 = 2.585 \times 10^{-3} moles}\)
\(\ce{n(HCl) = n(OH^{-})}\)
\[\ce{[OH-] = \frac{2.585 \times 10^{-3}}{0.02575} = 0.1004 mol L^{-1}}\]
b. \(\ce{n(HCl) = c \times V = 0.1004 \times 0.01655 = 1.661 \times 10^{-3} moles}\)
\(\ce{n(HCl) = n(C9H8O4) = 1.661 \times 10^{-3} moles}\)
\(\ce{MM(C9H8O4) = 9 \times 12.01 + 8 \times 1.008 + 4 \times 16.00 = 180.154 g}\)
\(\ce{Average mass of C9H8O4 per tablet}\)
\(\ce{= n \times MM = 1.661 \times 10^{-3} \times 180.154 = 0.2992 g = 299.2 mg}\)
A student attempted to determine the concentration of a hydrochloric acid solution. The following steps were performed.
Step 1. A conical flask was rinsed with water.
Step 2. A 25.0 mL pipette was rinsed with water.
Step 3. The student filled the pipette with a standard sodium carbonate solution to the level shown in the diagram.

Step 4. The standard sodium carbonate solution in the pipette was transferred to the conical flask. The student ensured that all of the sodium carbonate solution was transferred to the conical flask by blowing through the pipette. Three drops of an appropriate indicator were added to the conical flask.
Step 5. A burette was rinsed with the hydrochloric acid solution and then filled with the acid. The student then carried out a titration to determine the concentration of the hydrochloric acid solution.
In steps 2,3 and 4 above the student did not follow acceptable procedures.
a. Mistake: blowing through the pipette
→ Proposed change: student should have touched the end of the pipette to the surface of flask to draw out the liquid.
b. Mistake (step 2): rinsing the pipette with water
→ This would decrease the number of moles of \(\ce{Na2CO3}\) it contains.
Mistake (step 3): not filling to the gradation mark
→ By not filling to the mark, the pipette would contain fewer moles of \(\ce{Na2CO3}\).
→ Hence, a lower volume of the \(\ce{HCl}\) would be added from the burette, but the student would think that there were more moles of \(\ce{HCl}\) in this volume.
→ As a result, the calculated concentration of the acid solution would be higher than the actual concentration.
a. Mistake: blowing through the pipette.
→ Proposed change: student should have touched the end of the pipette to the surface of flask to draw out the liquid.
b. Mistake (step 2): rinsing the pipette with water
→ This would decrease the number of moles of \(\ce{Na2CO3}\) it contains.
Mistake (step 3): not filling to the gradation mark
→ By not filling to the mark, the pipette would contain fewer moles of \(\ce{Na2CO3}\).
→ Hence, a lower volume of the \(\ce{HCl}\) would be added from the burette, but the student would think that there were more moles of \(\ce{HCl}\) in this volume.
→ As a result, the calculated concentration of the acid solution would be higher than the actual concentration.
A solution was made by mixing 75.00 mL of 0.120 mol L\(^{-1}\) hydrochloric acid with 25.00 mL of 0.200 mol L\(^{-1}\) sodium hydroxide.
What is the pH of the solution? (3 marks)
\(\ce{pH = 1.4}\)
\(\ce{HCl + NaOH \rightarrow NaCl + H2O}\)
\(\ce{n(H3O+) = c \times V = 0.120 \times 0.07500 = 0.00900 moles}\)
\(\ce{n(OH) = c \times V = 0.02500 \times 0.200 = 0.00500 moles}\)
\(\ce{n(H3O+ excess) = 9 \times 10^{-3}-5 \times 10^{-3} = 4 \times 10^{-3} moles}\)
\[\ce{[H3O+] = \frac{4 \times 10^{-3}}{0.100} = 4.00 \times 10^{-2}}\]
\(\ce{pH = -log_10[H3O+] = -log_10 4.00 \times 10^{-2} = 1.4}\)
The nitrogen content of bread was determined using the following procedure:
--- 4 WORK AREA LINES (style=lined) ---
--- 2 WORK AREA LINES (style=lined) ---
--- 4 WORK AREA LINES (style=lined) ---
--- 4 WORK AREA LINES (style=lined) ---
a. \(\ce{NH3(g) + HCl(aq) \rightarrow NH4+(aq) + Cl-(aq)}\)
\(\ce{NaOH(aq) + HCl(aq) \rightarrow NaCl(aq) + H2O(l)}\)
b. \(2.70 \times 10^{-3}\)
c. \(3.55 \times 10^{-3}\)
d. \(1.78\%\)
a. \(\ce{NH3(g) + HCl(aq) \rightarrow NH4+(aq) + Cl-(aq)}\)
\(\ce{NaOH(aq) + HCl(aq) \rightarrow NaCl(aq) + H2O(l)}\)
b. \(\ce{n(NaOH excess) = c \times V = 0.116 \times 0.02330 = 2.70 \times 10^{-3} moles}\)
c. \(\ce{n(HCl original) = c \times V = 0.125 \times 0.0500 = 6.25 \times 10^{-3} moles}\)
\(\ce{n(HCl used) = 6.25 \times 10^{-3}-2.70 \times 10^{-3} = 3.55 \times 10^{-3} moles}\)
\(\ce{n(NH3) = n(HCl used) = 3.55 \times 10^{-3} moles}\)
d. \(\ce{n(N) = n(NH3) = 3.55 \times 10^{-3} moles}\)
\(\ce{Mass N = 3.55 \times 10^{-3} \times 14.01 = 0.0497 g}\)
\[\ce{\% N (by mass) = \frac{0.0497}{2.80} \times 100\% = 1.78\%}\]
In a fermentation experiment 6.50 g of glucose was completely converted to ethanol and carbon dioxide.
\(\ce{C6H12O6 \rightarrow 2C2H5OH + 2CO2}\)
What is the mass of carbon dioxide produced?
\(B\)
| \(MM(\ce{C6H12O6})\) | \(= 6(12.01)+12(1.008)+6(16)\) | |
| \(=180.156\ \text{gmol}^{-1}\) |
\(n(\ce{C6H12O6})=\dfrac{6.50}{180.156}=0.036\ \text{mol}\)
\(n(\ce{CO2})=0.072\ \text{mol}\)
\(m(\ce{CO2})=0.072 \times 44.01=3.18\ \text{g}\)
\(\Rightarrow B\)
Equal volumes of four 0.1 mol L\(^{-1}\) acids were titrated with the same sodium hydroxide solution.
Which one requires the greatest volume of base to change the colour of the indicator?
\(A\)
→ Citric acid is triprotic.
→ Sulfuric acid is diprotic.
→ Acetic acid and Hydrochloric acid are monoprotic.
→ The greatest volume of \(\ce{NaOH}\) is required for the triprotic acid as it has a 1:3, acid:base, stoichiometric ratio.
\(\Rightarrow A\)
A gas is produced when 10.0 g of zinc is placed in 0.50 L of 0.20 mol L\(^ {-1}\) nitric acid.
Calculate the volume of gas produced at 25°C and 100 kPa. Include a balanced chemical equation in your answer. (4 marks)
\(1.24\ \text{L}\)
\(\ce{2HNO3 + Zn \rightarrow Zn(NO3)2 + H2}\)
\(\ce{n(HNO3) = c \times V = 0.20 \times 0.5 = 0.10 moles}\)
\(\ce{n(Zn) = \dfrac{\text{m}}{\ce{MM}} = \dfrac{10}{65.38} = 0.153 moles}\)
→ \(\ce{HNO3}\) and \(\ce{Zn}\) react in a \(2:1\) ratio
→ \(\ce{HNO3}\) is the limiting reagent and 0.05 moles of gas \(\ce{H2}\) will be produced
\(\ce{V(H2) = 0.05 \times 24.79 = 1.24\ \text{L (2 d.p.)}}\)
\begin{array} {|c|c|}
\hline & \textit{Heat of} \\ \ \ \ \textit{Fuel}\ \ \ & \textit{combustion} \\ & (\text{kJ g}^{-1}) \\
\hline A & -48 \\ B & -38 \\ C & -28 \\
\hline \end{array}
--- 4 WORK AREA LINES (style=lined) ---
a. \(\ce{C4H9OH + 6O2 \rightarrow 4CO2 + 5H2O}\)
b. \(\text{Convert heat of combustion for each fuel to kJ mol}^{-1}:\)
\(\ce{MM(C4H9OH) = 12.01 \times 4 + 1.008 \times 9 + 16 + 1.008 = 74.12}\)
\(\ce{$A$: 48 \times 74 = 3552 kJ mol^{-1}}\)
\(\ce{$B$: 38 \times 74 = 2812 kJ mol^{-1}}\)
\(\ce{$C$: 28 \times 74 = 2072 kJ mol^{-1}}\)
\(\text{Energy will be lost due to heat and fuel impurities.}\)
\(A\ \text{and}\ B\ \text{have values higher than the published value.}\)
\(\therefore\ C\ \text{is most likely to be 1-butanol.}\)
a. \(\ce{C4H9OH + 6O2 \rightarrow 4CO2 + 5H2O}\)
b. \(\text{Convert heat of combustion for each fuel to kJ mol}^{-1}:\)
\(\ce{MM(C4H9OH) = 12.01 \times 4 + 1.008 \times 9 + 16 + 1.008 = 74.12}\)
\(\ce{$A$: 48 \times 74 = 3552 kJ mol^{-1}}\)
\(\ce{$B$: 38 \times 74 = 2812 kJ mol^{-1}}\)
\(\ce{$C$: 28 \times 74 = 2072 kJ mol^{-1}}\)
\(\text{Energy will be lost due to heat and fuel impurities.}\)
\(A\ \text{and}\ B\ \text{have values higher than the published value.}\)
\(\therefore\ C\ \text{is most likely to be 1-butanol.}\)
The heat of combustion of propan-1-ol is 2021 kJ mol\(^{-1}\). Combustion takes place according to the equation:
\( \ce{2C3H7OH}(l)+ \ce{9O2}(g) \rightarrow \ce{6CO2}(g) + \ce{8H2O}(l)\)
What mass of water is formed when 1530 kJ of energy is released?
\(C\)
\(\ce{n(C3H7OH)}=\dfrac{1530}{2021}=0.757\ \text{mol}\)
\(\ce{n(H2O)}=0.757 \times 4=3.03\ \text{mol}\)
\(\ce{m(H2O)}=3.03 \times 18.016=55\ \text{g}\)
\(\Rightarrow C\)
Sodium reacts with water to give hydrogen gas and sodium hydroxide solution.
What volume of gas would be produced from the reaction of 22.99 g of sodium at 25°C and 100 kPa ?
\(B\)
\(\ce{2Na(s) + 2H2O(l) \rightarrow H2(g) + 2NaOH(aq)}\)
\(n(\ce{Na(s)}) = \dfrac{22.99}{22.99}= 1\ \text{mol}\)
\(n(\ce{H2(g)}) = \dfrac{1}{2} \times 1= 0.5\ \text{mol}\)
The volume of \(\ce{H2(g)}= 0.5 \times 24.79 = 12.40\ \text{L}\)
\(\Rightarrow B\)
A batch of dry ice (solid \(\ce{CO_2}\)) was contaminated during manufacture. To determine its purity, the following steps were carried out.
a. 0.0500 moles
b. 80.0%
a. \(\ce{n(NaOH) = c \times V = 0.0500 \times 1.00 = 0.0500 moles}\)
b. \(\ce{n(HCl) = c \times V = 0.0276 \times 1.00 = 0.0276 mol (titrate excess NaOH)}\)
\(\ce{n(NaOH to neutralise CO2) = 0.0500-0.0276 = 0.0224 mol}\)
\(\ce{Ratio \ NaOH\ : CO2 = 2\ : 1 (from equation)}\)
\(\ce{n(CO2) = \frac{1}{2} \times n(NaOH) = \frac{1}{2} \times 0.0224 = 0.0112 mol}\)
\(\ce{MM (CO2) = 12.01 + 2 \times 16 = 44.01}\)
\(\ce{Mass (CO2) = n \times MM = 0.0112 \times 44.01 = 0.493 g}\)
\[\ce{\% Dry ice (by mass) = \frac{0.493}{0.616} \times 100\% = 80.0\%}\]
Under conditions of low oxygen levels, octane can undergo incomplete combustion according to the following chemical equation:
\( \ce{2C8H18}(l) + \ce{17O2}(g) \rightarrow \ce{6C}(s)+4 \ce{CO}(g) + \ce{6CO2}(g) + \ce{18H2O}(l)\)
Calculate the mass of soot \((\ce{C}(s))\) produced if 50 grams of octane are combusted in this way with 30 grams of oxygen and the mass of soot accounts for \(\dfrac{1}{5}\) of the total mass of the products. (2 marks)
\(16\ \text{g}\)
→ Total mass of the reactants 80 grams.
→ By the law of conservation of mass, the total mass of the products will be 80 grams.
→ Mass of soot \(=\dfrac{1}{5} \times 80 = 16\ \text{g}\)
What is the density of ozone \(\ce{(O3(g))}\) at 25°C and 100 kPa ?
\(C\)
→ Molar Mass of ozone \(= 3 \times 16=48\ \text{gmol}^{-1}\).
→ Density at 25°C and 100 kPa is 24.79 \(\text{Lmol}^{-1}\)
→ Density in \(\text{gL}^{-1}= \dfrac{48}{24.79}=1.936\ \text{gL}^{-1}\)
\(\Rightarrow C\)
The sodium hydroxide solution was mixed with 25.0 mL samples of 0.100 mol L\(^{-1}\) citric acid \(\ce{(C6H8O7)}\). The average volume of sodium hydroxide used was 41.50 mL.
\(\ce{C6H8O7 + 3NaOH \rightarrow C6H5O7Na3 + 3 H2O}\)
Calculate the concentration of the sodium hydroxide solution. (4 marks)
\(\ce{0.18 mol L^{-1} \text{(to 2 d.p.)}}\)
\(\ce{n(C6H8O7) = c \times V = 0.100 \times 0.0250 = 0.00250 moles}\)
\(\ce{NaOH : C6H8O7}\ \ \text{reaction ratio is}\ 3:1\)
\(\ce{n(NaOH in 41.50 mL) = 3 \times 0.00250 = 0.00750 moles}\)
\[\ce{[NaOH] = \frac{n}{V} = \frac{0.00750}{0.04150} = 0.18072… = 0.18 mol L^{-1} \text{(to 2 d.p.)}}\]
A solution of hydrochloric acid was standardised by titration against a sodium carbonate solution using the following procedure.
The titration was performed and the hydrochloric acid was found to be 0.200 mol L\(^{-1} \).
a. Substance for rinse:
→ Water should be used to rinse the conical flask as this will not change the number of moles of \(\ce{Na2CO3}\) placed in it.
b. \( 54.3\% \)
a. Substance for rinse:
→ Water should be used to rinse the conical flask as this will not change the number of moles of \(\ce{Na2CO3}\) placed in it.
b. \(\ce{HCl + NaOH \rightarrow H2O + NaCl}\)
\(\ce{n(NaOH) = c \times V = 0.250 \times 0.0295 = 7.375 \times 10^{-3} moles}\)
\(\ce{n(HCl) = 7.375 \times 10^{-3} (after reaction)}\)
\(\ce{n(HCl – original) = c \times V = 0.200 \times 0.0500 = 0.0100 moles}\)
\(\ce{n(HCl – used) = 0.0100-7.375 \times 10^{-3} = 2.625 \times 10^{-3} moles}\)
\(\ce{2HCl + CO3^{2-} \rightarrow H2O + CO2 + 2Cl-}\)
\(\ce{HCl\ : CO3^{2-} = 2\ : 1}\)
\[\ce{n(CO3^{2-}) = \frac{2.625 \times 10^{-3}}{2} = 1.3125 \times 10^{-3} moles}\]
\(\ce{m(CO3^{2-}) = 1.3125 \times 10^{-3} \times 60.01 = 0.07876 g}\)
\[\ce{\text{% Mass} (CO3^{2-}) = \frac{0.07876}{0.145} \times 100\% = 54.3\%} \]
Three electron configurations are presented in the table. For elemental titanium, the ground state is represented by \(\text{I}\), while \(\text{II}\) and \(\text{III}\) are both invalid ground state electron configurations.
Write a valid electron configuration for \(\ce{Ti^3+}\). (2 marks)
Possible answer structures include:
\(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 3d^1}\)
![]()
Possible answer structures include:
\(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 3d^1}\)
![]()
An unattended car is stationary with its engine running in a closed workshop. The workshop is 5.0 m × 5.0 m × 4.0 m and its volume is \(1.0\ ×\ 10^{5}\) L. The engine of the car is producing carbon monoxide in an incomplete combustion according to the following chemical equation:
\(\ce{C8H18(l)+\dfrac{17}{2}O2(g) \rightarrow 8CO(g) + 9H2O(l)}\)
Exposure to carbon monoxide at levels greater than 0.100 g L\(^{-1}\) of air can be dangerous to human health.
6.0 kg of octane was combusted by the car in this workshop.
Using the equation provided, determine if the level of carbon monoxide produced in the workshop would be dangerous to human health. Support your answer with relevant calculations. (4 marks)
\(\ce{\text{Volume of garage} = 5 \times 5 \times 4 = 100 \text{m}^2}\)
\(\ce{100 \text{m}^2} = 100\ 000\ \text{L}\)
\[\ce{\text{n(octane)} = \frac{n}{M} = \frac{6000}{114.224} = 52.53 \text{moles}} \]
\(\ce{Molar ratio of Octane : CO = 1:8}\)
\(\ce{n(CO) = 8 \times 52.53 = 420.23}\)
\(\ce{m(CO) = n \times M = 420.23 \times 28.01 = 11\ 771\ g}\)
\[\ce{[CO] = \frac{11\ 771}{100\ 000} = 0.118 g L^{-1}}\]
\(\ce{\text{Since} [CO] > 0.100 g L^{-1}, \text{it is dangerous to human health.}}\)
\(\ce{\text{Volume of garage} = 5 \times 5 \times 4 = 100 \text{m}^2}\)
\(\ce{100 \text{m}^2} = 100\ 000\ \text{L}\)
\[\ce{\text{n(octane)} = \frac{n}{M} = \frac{6000}{114.224} = 52.53 \text{moles}} \]
\(\ce{Molar ratio of Octane : CO = 1:8}\)
\(\ce{n(CO) = 8 \times 52.53 = 420.23}\)
\(\ce{m(CO) = n \times M = 420.23 \times 28.01 = 11\ 771\ g}\)
\[\ce{[CO] = \frac{11\ 771}{100\ 000} = 0.118 g L^{-1}}\]
\(\ce{\text{Since} [CO] > 0.100 g L^{-1}, \text{it is dangerous to human health.}}\)
Excess barium nitrate solution is added to 200 mL of 0.200 mol L\(^{-1}\) sodium sulfate.
What is the mass of the solid formed?
`C`
\( \ce{Ba(NO3)2 (aq) + Na2SO4 (aq) \rightarrow BaSO4 (s) + 2NaNO3 (aq)}\)
\(n(\ce{Na2SO4})=0.2 \times 0.2=0.04\ \text{mol}\)
\(n(\ce{BaSO4 (s)})=0.04\ \text{mol}\)
\(m(\ce{BaSO4 (s)})=0.04 \times 233.37=9.33\ \text{g}\)
\(\Rightarrow C\)
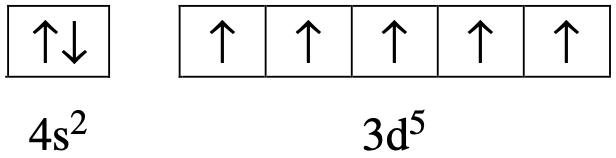
The electron spin orbital diagram represents the \(3d\) and \(4s\) electrons for an element in the first transition series.
Identify this element and explain the arrangement of electrons in these sub-shells in terms of the Pauli exclusion principle and Hund's rule. (3 marks)
→ The electron configuration of \(3d^{6}\) \(4s^{2}\) is that of Iron (Atomic No. 26).
→ Hund’s Rule: Every orbital within a sub shell must by singly occupied before an orbital can become doubly occupied.
→ Pauli Exclusion Principle: No more than 2 electrons can occupy the same orbital and the 2 electrons in the same orbital must have opposite spin.
→ The electron configuration of \(3d^{6}\) \(4s^{2}\) is that of Iron (Atomic No. 26).
→ Hund’s Rule: Every orbital within a sub shell must by singly occupied before an orbital can become doubly occupied.
→ Pauli Exclusion Principle: No more than 2 electrons can occupy the same orbital and the 2 electrons in the same orbital must have opposite spin.
The diagram below shows the ground state electron configuration of two complexes of cobalt in aqueous solution.
Identify the block in the periodic table to which cobalt belongs and write the electron configuration of cobalt metal in its ground state. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
→ Cobalt belongs to the D-block on the periodic table.
→ The electron configuration of Cobalt metal in its ground state is:
\(1s^{2}\) \(2s^{2}\) \(2p^{6}\) \(3s^{2}\) \(3p^{6}\) \(4s^{2}\) \(3d^{7}\)
→ Cobalt belongs to the D-block on the periodic table.
→ The electron configuration of Cobalt metal in its ground state is:
\(1s^{2}\) \(2s^{2}\) \(2p^{6}\) \(3s^{2}\) \(3p^{6}\) \(4s^{2}\) \(3d^{7}\)
Write the full electron configurations for a \(\ce{Ca}\) atom in the ground state, an excited \(\ce{Ca}\) atom and a \( \ce{Ca}^{+}\) ion. (2 marks)
\(\ce{Ca}\) atom: \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2}\)
\(\ce{Ca}\) atom (excited): \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 4p^1}\)
\(\ce{Ca^+}\) atom: \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s}\)
\(\ce{Ca}\) atom: \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2}\)
\(\ce{Ca}\) atom (excited): \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 4p^1}\)
\(\ce{Ca^+}\) atom: \(\ce{1s^2 2s^2 2p^6 3s^2 3p^6 4s}\)
Experimental evidence from emission line spectra of gaseous atoms has highlighted both the merits and the limitations of Bohr's atomic model.
Discuss Bohr's atomic model with reference to this evidence. (5 marks)
→ The Bohr model of the atom describes the orbit of electrons around the nucleus at fixed radii and energy.
→ The emission line spectrum of hydrogen was seen to support the Bohr model as discrete lines were observed which could be assigned to electronic transitions between fixed energy levels.
→ However when the emission line spectrum for sodium was recorded there were more spectral lines than could be explained by the Bohr model. Line splitting or doublets were observed.
→ The line splitting or doublets result from electrons having differing angular momenta, residing in sub-shells, which was not considered in the Bohr model.
Identify the element in period 3 of the periodic table that has the highest electronegativity and justify your choice. (3 marks)
→ Most electronegative element in period 3 is chlorine \(\ce{(Cl)}\).
→ The electronegativity is a measure of an atom’s ability to attract electrons to itself.
→ Electronegativities increase across periods in the periodic table from left to right or as the number of valence electrons increases.
→ Fluorine is the most electronegative element with all other electronegativities relative to this.
→ Most electronegative element in period 3 is chlorine \(\ce{(Cl)}\).
→ The electronegativity is a measure of an atom’s ability to attract electrons to itself.
→ Electronegativities increase across periods in the periodic table from left to right or as the number of valence electrons increases.
→ Fluorine is the most electronegative element with all other electronegativities relative to this.
`A`
→ A coordinate covalent bond occurs when one atom donates a pair of electrons (both electrons) to form the covalent bond.
→ In \(A\) both atoms donate 1 electron each, therefore it is not a coordinate covalent bond.
`=>A`
`B`
→ Ethanol, \(\ce{C2H5OH}\) has a polar \(\ce{OH}\) group. The partially negative oxygen atom is attracted to the partially positive hydrogen atom in the water molecule.
→ This forms a hydrogen bond.
`=>B`
Identify ONE cation and ONE anion that can be represented by the electron configuration \( \ce{1 s^2 2 s^2 2 p^6 3 s^2 3 p^6} \). (2 marks)
Answers could include one of the following:
\(\ce{K+, Cl-}\)
\(\ce{Ca^{2+}, S^{2-}}\)
\(\ce{Sc^{3+}, P^{3-}}\)
Answers could include one of the following:
\(\ce{K+, Cl-}\)
\(\ce{Ca^{2+}, S^{2-}}\)
\(\ce{Sc^{3+}, P^{3-}}\)
Element 112 was first synthesised in 1996 and officially named in 2009 as copernicium, \(\ce{Cn}\).
Explain why the transuranic isotope \( \ce{^{278 }_{112}Cn}\) is unstable. (1 mark)
--- 2 WORK AREA LINES (style=lined) ---
→ Isotopes such as copernicium-278 are unstable because they are heavy nuclei with high neutron-proton ratios (eg. Cn-278 = 166 : 112).
→ Isotopes such as copernicium-278 are unstable because they are heavy nuclei with high neutron-proton ratios (eg. Cn-278 = 166 : 112).
What property of \(\ce{O3}\) makes it more soluble in water than \(\ce{O2}\) in water?
`A`
→ Solubility in water is directly correlated to the polarity of a molecule. The more polar a molecule is the more it will dissolve in water.
→ Thus if \(\ce{O3}\) is more soluble in water than \(\ce{O2}\), it must be polar while \(\ce{O2}\) is relatively nonpolar.
`=>A`
Evaluate the contribution of the Bohr model to the development of our understanding of the structure of the atom. ( 7 marks)
→ The Bohr model of the atom was developed to use the quantisation of energy to explain the emission and absorption spectrum of hydrogen.
→ It proposed that the single electron of the atom was confined to defined orbits around the nucleus, somewhat analogous to a planet’s orbit around its star.
→ For the electron to change to a different orbit, energy had to be absorbed (to go to a higher energy orbit) or emitted (to go to a lower energy orbit).
→ The energy absorbed or emitted is well-defined, giving rise to sharp absorption or emission lines. No other changes in energy are allowed.
→ The model was an important step towards understanding and accepting the quantum view of the atom and introduced the idea of energy levels (shells) to describe the electronic configuration of atoms.
→ The Bohr model could not be applied to atoms other than hydrogen and did not provide any explanation for the quantisation.
→ These restrictions and limitations of the model were recognised by Bohr and the model was not meant to be used beyond the hydrogen atom, but the attractiveness of the simplicity of the model has ensured continued use and propagates the incorrect concept of electrons as particles orbiting a nucleus.
Write the electronic configuration of \(\ce{Fe^2+}\) and \(\ce{Fe^3+}\). (2 marks)
Method 1
\(\ce{Fe^2+: [Ar] \text{3d}^6 }\)
\(\ce{Fe^3+: [Ar] \text{3d}^5 }\)
Method 2
\(\ce{Fe^2+: 1s^2 2s^2 2p^2 2p^6 3s^2 3p^6 4s^2 3d^6}\)
\(\ce{Fe^3+: 1s^2 2s^2 2p^2 2p^6 3s^2 3p^6 4s^2 3d^5}\)
`C`
→ Acidic Oxides: are often the oxides of non-metals and form acidic solutions.
→ Basic Oxides: are usually formed by reacting oxygen with metals and participate with acids in neutralisation reactions.
→ Neutral Oxides: react with neither acids or bases and do not lead to either acidic or basic solutions.
→ Amphoteric Oxides: exhibit both acidic and basic properties and can chemically react as either an acid or base.
`=>C`
An atom has FIVE valence electrons in its \(d\) orbital. Using an orbital diagram of the valence shell of the atom, explain how Hund's rule determines the electronic configuration. (3 marks)
→ Hund’s rule states that every orbital in a subshell is occupied with one electron before any orbital is doubly occupied.
→ In this example Hund’s rule is illustrated by the \(3d\) sub-shell having 5 electrons in 5 different orbitals.

→ Hund’s rule states that every orbital in a subshell is occupied with one electron before any orbital is doubly occupied.
→ In this example Hund’s rule is illustrated by the \(3d\) sub-shell having 5 electrons in 5 different orbitals.
Identify in which of the \( s, p, d \) or \(f \) blocks of the Periodic Table the element \(\ce{Ra}\) is found. Justify your answer. (2 marks)
→ \(\ce{Ra}\) (radium) is an \( s \)-block element.
→ Its valence electrons are in the \( s \) orbital.
The emission spectrum of a metal salt is observed with a spectroscope.
Explain the processes by which emission lines arise. Include an energy level diagram for the lines marked A and B in your response. (3 marks)
→ The metal ions in the solution gain energy from the components of the flame and are electronically excited, ie electrons are promoted to higher energy states.
→ The electrons fall back to lower energy states, releasing energy as photons.
→ The lines observed through the spectroscope are therefore emission lines. Because the electron energies are quantised, and transitions between states are restricted to well-defined energies, the energy released is well defined, thus the emission is seen as lines and not a continuous spectrum.
→ Line A is the blue end of the spectrum, meaning the transition is of high energy (shorter wavelength).
→ Line B is at the red end of the spectrum, meaning the transition is of low energy (longer wavelength).
The structures of ozone and molecular oxygen are shown.
Ozone is more easily decomposed than molecular oxygen because
`D`
→ The presence of the single bond in the ozone molecule lowers the average bond energy.
→ Bond breaking is an endothermic process and so requires energy. As ozone has a lower average bond energy, less energy is required to break the bonds and thus decomposes more easily than molecular oxygen.
`=>D`
Demonstrate how applications of the Human Genome Project could affect future trends in human biological evolution. (4 marks)
→ The Human Genome Project has succeeded in mapping genes and identifying base sequences of the entire genome.
→ Precise locations of disease-causing genes have been discovered, as well as their specific base sequences.
→ Genetic screening allows people to find out whether they hold defective genes. This can show whether themselves, their family or potentially future children are at risk of a genetic disease even before symptoms appear.
→ Modification of lifestyle could help to prolong life and increase the chances of the individual producing offspring. This could increase the frequency of the defective gene in the population.
→ Pharmaceuticals can be designed to prevent expression of defective DNA using base sequence recognition chemistry.
→ This would mean that holders of a defective gene would not experience a defective genotype and might live longer lives, causing the defective gene to become more common in the humans species as a consequence.
→ CRISPR is an emerging gene-editing technology that can be used to modify, delete or correct precise regions of our DNA. Its use on humans is currently very limited but its potential is promising for treatment of genetic diseases.
→ While somatic gene editing by CRISPR affects only the patient being treated, germ-line editing affects all cells in an organism, including eggs and sperm. This means that future generations who would normally be affected by the genetic disease in question would be unaffected as the defective gene would not be part of their genotype.
→ The Human Genome Project has succeeded in mapping genes and identifying base sequences of the entire genome.
→ Precise locations of disease-causing genes have been discovered, as well as their specific base sequences.
→ Genetic screening allows people to find out whether they hold defective genes. This can show whether themselves, their family or potentially future children are at risk of a genetic disease even before symptoms appear.
→ Modification of lifestyle could help to prolong life and increase the chances of the individual producing offspring. This could increase the frequency of the defective gene in the population.
→ Pharmaceuticals can be designed to prevent expression of defective DNA using base sequence recognition chemistry.
→ This would mean that holders of a defective gene would not experience a defective genotype and might live longer lives, causing the defective gene to become more common in the humans species as a consequence.
→ CRISPR is an emerging gene-editing technology that can be used to modify, delete or correct precise regions of our DNA. Its use on humans is currently very limited but its potential is promising for treatment of genetic diseases.
→ While somatic gene editing by CRISPR affects only the patient being treated, germ-line editing affects all cells in an organism, including eggs and sperm. This means that future generations who would normally be affected by the genetic disease in question would be unaffected as the defective gene would not be part of their genotype.
'Science has been used to solve problems in the investigation of evolutionary
relationships between humans and other primates, and so has provided information of interest to society.'
Justify this statement in terms of the scientific knowledge behind DNA-DNA hybridisation AND karyotype analysis. (7 marks)
--- 20 WORK AREA LINES (style=lined) ---
→ Using comparative morphologies limits our ability in determining relationships between humans and other primates. Sometimes morphologies seem very different, yet the changes or modifications required to achieve those differences might be small in number or too simple.
→ Using genetic evidence gives a much more accurate picture.
DNA-DNA Hybridisation
→ Can be used to show how genetically similar two species are.
→ DNA from a human and a chimpanzee (other primate) can be tested for melting point. Then it can be melted into single strands. The single strands are combined into hybrid DNA, in which some hydrogen bonding between base pairs does not happen because they are not complementary.
→ The lower the hybrid DNA M.P. is compared to the original DNA is a measure of how similar the original DNA was.
→ When the DNA is similar the two species are seen to be close in evolutionary terms.
Karyotype Analysis
→ Involves using a chemical to kill a cell during cell division when the chromosomes can be seen individually.
→ Photos are taken and the chromosome pictures arranged in pairs of increasing size. This picture of all the chromosomes in the genome is a karyotype.
→ Comparing the number, size, shape and banding pattern of chromosomes allows scientists to observe differences between species.
→ The fewer differences between karyotypes, the closer the species are in evolutionary terms.
→ People are interested to study our closest living relatives, as it helps us to understand where we have come from. It helps us to understand ourselves as a species when we can identify our closest living relatives and see our unique or common features and behaviours. DNA-DNA hybridisation and karyotype analysis help scientists to accurately achieve this knowledge.
→ Using comparative morphologies limits our ability in determining relationships between humans and other primates. Sometimes morphologies seem very different, yet the changes or modifications required to achieve those differences might be small in number or too simple.
→ Using genetic evidence gives a much more accurate picture.
DNA-DNA Hybridisation
→ Can be used to show how genetically similar two species are.
→ DNA from a human and a chimpanzee (other primate) can be tested for melting point. Then it can be melted into single strands. The single strands are combined into hybrid DNA, in which some hydrogen bonding between base pairs does not happen because they are not complementary.
→ The lower the hybrid DNA M.P. is compared to the original DNA is a measure of how similar the original DNA was.
→ When the DNA is similar the two species are seen to be close in evolutionary terms.
Karyotype Analysis
→ Involves using a chemical to kill a cell during cell division when the chromosomes can be seen individually.
→ Photos are taken and the chromosome pictures arranged in pairs of increasing size. This picture of all the chromosomes in the genome is a karyotype.
→ Comparing the number, size, shape and banding pattern of chromosomes allows scientists to observe differences between species.
→ The fewer differences between karyotypes, the closer the species are in evolutionary terms.
→ People are interested to study our closest living relatives, as it helps us to understand where we have come from. It helps us to understand ourselves as a species when we can identify our closest living relatives and see our unique or common features and behaviours. DNA-DNA hybridisation and karyotype analysis help scientists to accurately achieve this knowledge.
--- 4 WORK AREA LINES (style=lined) ---
The students concluded that their data were conflicting and they could not determine the relative ages of the fossils.
Evaluate the students' conclusion with reference to the data presented. (4 marks)
--- 8 WORK AREA LINES (style=lined) ---
i. Relative vs absolute dating:
→ Relative dating is used to compare fossils, giving scientists data to determine if one fossil is older/younger than another.
→ Absolute dating can provide a quantitative value for the actual age of individual fossils.
ii. Evaluation of conclusion:
→ Fossil B is located at a lower depth than fossil A, however, the strata that contains fossil A is below the strata that contains fossil B.
→ This has occurred because the landscape was folded via geological processes, pushing up some of the strata containing fossil A and pushing down the strata containing fossil B. Therefore, fossil A is the older of the two.
→ Fossil C is younger because it is located in a higher strata than the other two fossils.
→ In Figure 2, fossil A is shown to have an age of four half-lives of \(\ce{C^{14}}\), fossil B three half-lives and fossil C one half-life. This evidence suggests fossil A is the oldest and fossil C is much younger.
→ Therefore the students’ conclusion is incorrect as the graphical data is consistent with the mapped data.
i. Relative vs absolute dating:
→ Relative dating is used to compare fossils, giving scientists data to determine if one fossil is older/younger than another.
→ Absolute dating can provide a quantitative value for the actual age of individual fossils.
ii. Evaluation of conclusion:
→ Fossil B is located at a lower depth than fossil A, however, the strata that contains fossil A is below the strata that contains fossil B.
→ This has occurred because the landscape was folded via geological processes, pushing up some of the strata containing fossil A and pushing down the strata containing fossil B. Therefore, fossil A is the older of the two.
→ Fossil C is younger because it is located in a higher strata than the other two fossils.
→ In Figure 2, fossil A is shown to have an age of four half-lives of \(\ce{C^{14}}\), fossil B three half-lives and fossil C one half-life. This evidence suggests fossil A is the oldest and fossil C is much younger.
→ Therefore the students’ conclusion is incorrect as the graphical data is consistent with the mapped data.
The diagram shows two primates. --- 2 WORK AREA LINES (style=lined) --- --- 5 WORK AREA LINES (style=lined) --- i. Figure 1: Prehensile Figure 2: Non-prehensile ii. → Classification will be identical in higher levels, not necessarily the same in the lower levels. → Since phylum is above order in the classification system, these two animals in the same order will be in the same phylum. → Genus is below order in the classification system, so these animals are not necessarily in the same genus. i. Figure 1: Prehensile Figure 2: Non-prehensile ii. → Classification will be identical in higher levels, not necessarily the same in the lower levels. → Since phylum is above order in the classification system, these two animals in the same order will be in the same phylum. → Genus is below order in the classification system, so these animals are not necessarily in the same genus.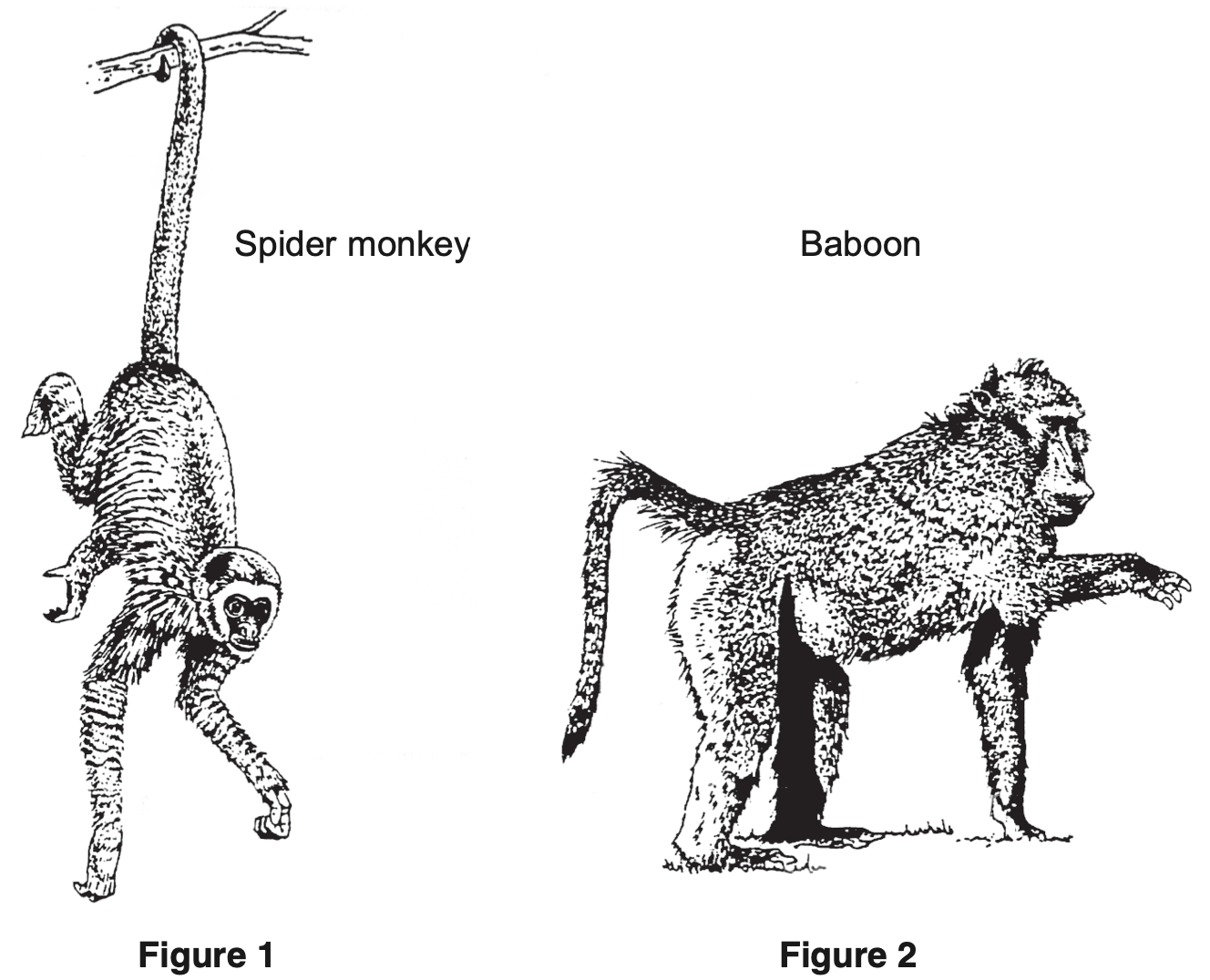
A new fossil form was recently found in South Africa. This fossil shares characteristics with both the genus Australopithecus and the genus Homo. There has been debate as to whether this new fossil form should be classified in the genus Australopithecus or in the genus Homo. --- 3 WORK AREA LINES (style=lined) --- --- 8 WORK AREA LINES (style=lined) --- i. → If the fossil is to be classified as the genus Homo, then the fossil should indicate an upright stance. → Alternatively, if the fossil is to be classified as the genus Australopithecus, then the fossil should indicate a stooped stance. ii. → Mitochondrial DNA sequences of the fossil and a modern Homo species (eg Homo sapiens) could be compared to determine time since a common ancestor. → If the time since a common ancestor is less than 2 MYA, the fossil is likely to be of the Homo genus. → If it is greater than 2 MYA since a common ancestor, it is likely that this fossil is either Australopithecus or another species. i. → Genus Homo: the fossil should indicate an upright stance. → Genus Australopithecus: the fossil should indicate a stooped stance. ii. DNA sequencing → Mitochondrial DNA sequences of the fossil and a modern Homo species (eg Homo sapiens) could be compared to determine time since a common ancestor. → If the time since a common ancestor is less than 2 MYA, the fossil is likely to be of the Homo genus. → If it is greater than 2MYA since a common ancestor, it is likely that this fossil is either Australopithecus or another species.
The table compares some of the amino acids present in a particular protein in different primates.
Using these data and your knowledge of the characteristics of primate groups, explain why using different types of data improves the reliability of estimated evolutionary relationships. (5 marks)
→ The amino acid data set shows that chimpanzees and humans have identical amino acids in this protein.
→ Gorillas show one amino acid difference, new world monkeys show three amino acid differences and prosimians show four amino acid differences.
→ On the basis of this data, it can be assessed that chimpanzees and humans are identical, followed by gorillas then new world monkeys and then prosimians.
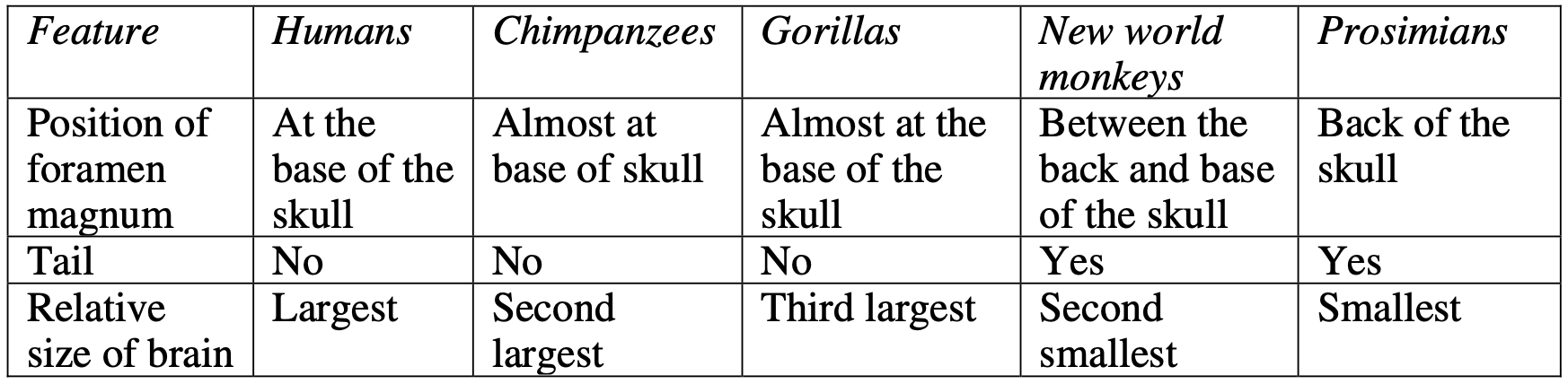
→ The morphological characteristics outlined in the table would rank the organisms in evolutionary proximity as chimpanzees most closely related to humans but different species, followed by gorillas then new world monkeys and then prosimians.
→ Both data sets correlate and therefore the estimates of evolutionary proximity to humans can be considered to be more reliable.
--- 2 WORK AREA LINES (style=lined) ---
--- 8 WORK AREA LINES (style=blank) ---
i. → The position of centromeres.
→ The banding patterns present.
ii.

i. → The position of centromeres
→ The banding patterns present.
ii.

In 1926, T H Muller experimented with fruit flies (Drosophila sp.) by exposing them to X-rays. He found that their offspring showed new phenotypes not observed in the wild population.
Explain how the results of these experiments can provide support for Darwin's theory of evolution by natural selection. (4 marks)
--- 9 WORK AREA LINES (style=lined) ---
→ He demonstrated that genetic mutations produced by X-rays in the lab, could be passed on to offspring.
→ As the X-rays could induce genetic diversity in the fruit flies, Muller’s experiments proved that genetic variation could be increased.
→ These findings bridged the gap between laboratory experiments and field observations, making evolution a rigorous experimental science
→ Muller’s work provided experimental evidence that genetic mutations could drive evolutionary change, aligning with Darwin’s theory.
→ He demonstrated that genetic mutations produced by X-rays in the lab, could be passed on to offspring.
→ As the X-rays could induce genetic diversity in the fruit flies, Muller’s experiments proved that genetic variation could be increased.
→ These findings bridged the gap between laboratory experiments and field observations, making evolution a rigorous experimental science
→ Muller’s work provided experimental evidence that genetic mutations could drive evolutionary change, aligning with Darwin’s theory.
--- 2 WORK AREA LINES (style=lined) ---
--- 7 WORK AREA LINES (style=lined) ---
a. Punctuated equilibrium:
→ Once species adapts to their environment, they will undergo little or no evolutionary change, remaining in an equilibrium state until a rapid change to their environment forces evolutionary change.
b. Punctuated equilibrium vs Darwin:
→ These two theories both acknowledge natural selection, but differ in the pace and pattern of evolution.
→ Darwin’s theory suggests gradual, continuous evolution, with small changes accumulating over time as species adapt to their environment.
→ In contrast, punctuated equilibrium proposes that species remain stable for long periods with drastic evolutionary changes occurring in when the environment undergoes rapid change, thus forcing quick evolutionary change.
a. Punctuated equilibrium:
→ Once species adapts to their environment, they will undergo little or no evolutionary change, remaining in an equilibrium state until a rapid change to their environment forces evolutionary change.
b. Punctuated equilibrium vs Darwin:
→ These two theories both acknowledge natural selection, but differ in the pace and pattern of evolution.
→ Darwin’s theory suggests gradual, continuous evolution, with small changes accumulating over time as species adapt to their environment.
→ In contrast, punctuated equilibrium proposes that species remain stable for long periods with drastic evolutionary changes occurring in when the environment undergoes rapid change, thus forcing quick evolutionary change.
The widespread use of antibiotics for the treatment of bacterial infections has led to the development of antibiotic resistance in some species of bacteria. From your studies of evolution and the mechanisms of inheritance, explain how resistance has developed in bacteria. (3 marks)
--- 6 WORK AREA LINES (style=lined) ---
→ Resistance in bacteria develops through a process of natural selection and genetic mutation.
→ Some bacteria possess random mutations that allow them to be resistant to antibiotics.
→ As these resistant bacteria are more likely to survive, when they reproduce via binary fission, the daughter cells will also possess the resistant mutation.
→ Over time, the whole population of bacteria becomes antibiotic-resistant because the resistant bacteria are best suited to their environment. This is how resistance has developed in bacteria.
→ Resistance in bacteria develops through a process of natural selection and genetic mutation.
→ Some bacteria possess random mutations that allow them to be resistant to antibiotics.
→ As these resistant bacteria are more likely to survive, when they reproduce via binary fission, the daughter cells will also possess the resistant mutation.
→ Over time, the whole population of bacteria becomes antibiotic-resistant because the resistant bacteria are best suited to their environment. This is how resistance has developed in bacteria.
Justify the use of vertebrate forelimbs as evidence to support the theory of evolution. (3 marks) --- 7 WORK AREA LINES (style=lined) --- → Vertebrate forelimbs exhibit a shared structure across species, suggesting common ancestry. → Despite varying functions and appearances, the five-fingered bone structure remains consistent. → The transition from fins to limbs in early tetrapods further supports evolution as these transitions reveal functional stages in limb development. → By comparing the limbs of vertebrate we are presented with compelling evidence to support the theory of evolution. → Vertebrate forelimbs exhibit a shared structure across species, suggesting common ancestry. → Despite varying functions and appearances, the five-fingered bone structure remains consistent. → The transition from fins to limbs in early tetrapods further supports evolution as these transitions reveal functional stages in limb development. → By comparing the limbs of vertebrate we are presented with compelling evidence to support the theory of evolution.