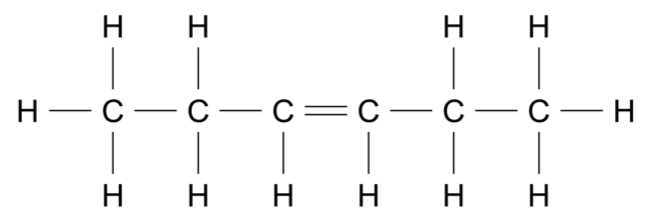
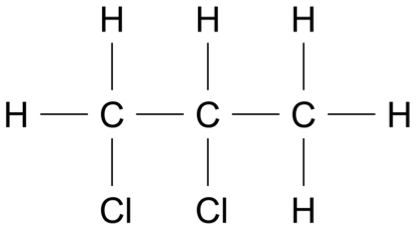
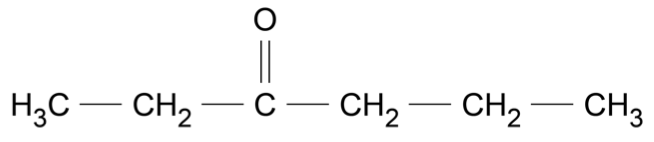If 20.0 g of methane gas, \(\ce{CH4}\), is kept in a 5.0 L sealed container at 25 °C, what would be the pressure in the container? (2 marks)
CHEMISTRY, M2 2016 VCE 9a
Standard solutions of sodium hydroxide, \(\ce{NaOH}\), must be kept in airtight containers. This is because \(\ce{NaOH}\) is a strong base and absorbs acidic oxides, such as carbon dioxide, \(\ce{CO2}\), from the air and reacts with them. As a result, the concentration of \(\ce{NaOH}\) is changed to an unknown extent.
\(\ce{CO2}\) in the air reacts with water to form carbonic acid, \(\ce{H2CO3}\). This can react with \(\ce{NaOH}\) to form sodium carbonate, \(\ce{Na2CO3}\).
- Write a balanced overall equation for the reaction between \(\ce{CO2}\) gas and water to form \(\ce{H2CO3}\). (1 mark)
--- 1 WORK AREA LINES (style=lined) ---
- Write a balanced equation for the complete reaction between \(\ce{H2CO3}\) and \(\ce{NaOH}\) to form \(\ce{Na2CO3}\). (1 mark)
--- 1 WORK AREA LINES (style=lined) ---
CHEMISTRY, M7 2016 VCE 12 MC
A condensation reaction involving 200 glucose molecules, \(\ce{C6H12O6}\), results in a polysaccharide. The molar mass, in g mol\(^{-1}\), of the polysaccharide is
- 36 000
- 35 982
- 32 418
- 32 400
PHYSICS, M1 2013 HSC 22
This set of data was obtained from a motion investigation to determine the acceleration due to gravity on a planet other than Earth. --- 5 WORK AREA LINES (style=lined) ---
Time (s)
Vertical velocity (m s\(^{-1}\))
0.60
0.02
1.00
0.09
1.20
0.12
1.40
0.17
1.80
0.23
Plot the data from the table, and then calculate the acceleration. (3 marks)
CHEMISTRY, M2 2018 VCE 1
Industrially, ethanol, \(\ce{C2H5OH} \), is made by either of two methods.
One method uses ethene, \(\ce{C2H4} \), which is derived from crude oil.
The other method uses a sugar, such as sucrose, \(\ce{C12H22O11}\), and yeast, in aqueous solution.
The production of \(\ce{C2H5OH}\) from \(\ce{C12H22O11}\) and yeast proceeds according to the equation
\( \ce{C12H22O11(aq) + H2O(l) \rightarrow 4C2H5OH(aq) + 4CO2(g)} \)
- Determine the mass, in grams, of pure \(\ce{C2H5OH}\) that would be produced from 1.250 kg of \(\ce{C12H22O11}\) dissolved in water.
\(\ce{MM(C12H22O11) = 342\ \text{g mol}^{-1} }\) (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
- i. Complete the reaction by writing the formula for the reactant in the box provided below.
\(\ce{C2H4(g) + \text{insert box} ->[\text{catalyst}] C2H5OH(g) }\) (1 mark)
--- 5 WORK AREA LINES (style=lined) ---
- ii. Classify this type of reaction. (1 mark)
--- 5 WORK AREA LINES (style=lined) ---
PHYSICS, M3 2013 HSC 33b
Outline a first-hand investigation to demonstrate the transfer of light by optical fibres. (2 marks) --- 6 WORK AREA LINES (style=lined) ---
PHYSICS, M4 2013 HSC 26a
CHEMISTRY, M7 2023 HSC 15 MC
The table gives the heat of combustion of three different alcohols at 25°C.
| \( Alcohol \) | \( Heat \ of \ combustion \) \( \text{(Kj g} ^{-1}) \) |
| \( \text{Methanol} \) | \(22.68\) |
| \( \text{Ethanol} \) | \(29.67\) |
| \( \text{Butan-1-ol} \) | \(36.11\) |
Which of the following gives the best approximation for the molar heat of combustion of propan-1-ol, expressed in kJ g\(^{-1} \)?
- \(\left(\dfrac{22.68+29.67+36.11}{3}\right)\)
- \(\left(\dfrac{29.67+36.11}{2}\right)\)
- \(\left(\dfrac{22.68+29.67}{2}\right)\)
- \(\left(\dfrac{3 \times 36.11}{4}\right)\)
CHEMISTRY, M8 2023 HSC 13 MC
The table shows four separate tests used to identify a dilute, aqueous sample of a compound.
\begin{array} {|c|l|l|}
\hline
\rule{0pt}{2.5ex} \textbf{Test Number} \rule[-1ex]{0pt}{0pt} & \quad \quad \quad \quad \quad \textbf{Test} & \quad \quad \quad \textbf{Observation}\\
\hline
\rule{0pt}{2.5ex} 1\rule[-1ex]{0pt}{0pt} & \text{Test with Red litmus} & \text{Stays red}\\
\hline
\rule{0pt}{2.5ex} 2 \rule[-1ex]{0pt}{0pt} & \text{Add \( \ce{Ba}^{2+} \) ions to a sample} & \text{White precipitate formed}\\
\hline
\rule{0pt}{2.5ex} 3 \rule[-1ex]{0pt}{0pt} & \text{Add \( \ce{OH}^{-} \) ions to a sample}& \text{Brown precipitate formed} \\
\hline
\rule{0pt}{2.5ex} 4 \rule[-1ex]{0pt}{0pt} & \text{Add \( \ce{Cl}^{-}\) ions to a sample}& \text{White precipitate formed}\\
\hline
\end{array}
Which compound would produce the observations shown?
- Silver sulfate
- Lead(\(\text{II}\)) acetate
- Iron(\(\text{II}\)) bromide
- Magnesium carbonate
CHEMISTRY, M6 2023 HSC 11 MC
An indicator solution was obtained by boiling a flower in water.
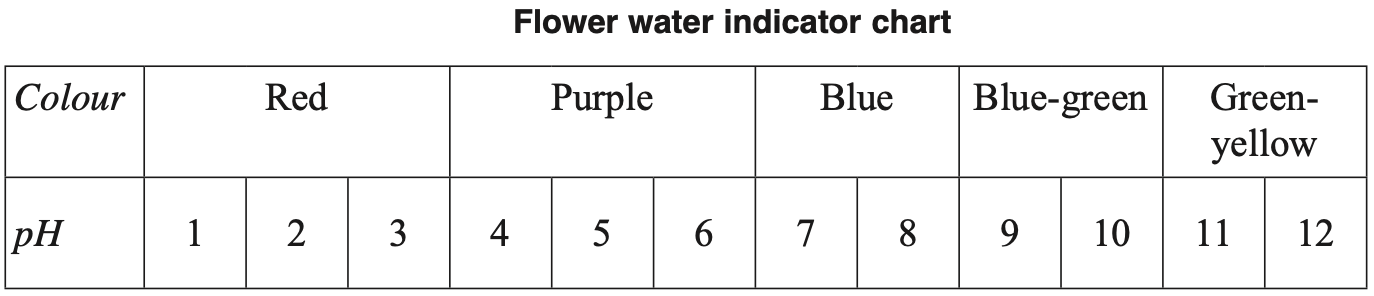
Two solutions were tested with this indicator.
Which row of the table correctly identifies the colour of each solution?
| \( \ce{H2SO4} \ (1 \ × \ 10^{-5} \ \text{mol L}^{-1}) \) | \( \ce{NaOH} \ (5 \ × \ 10^{-5} \ \text{mol L}^{-1}) \) | |
| \(\text{A.}\) | \( \text{Red} \) | \( \text{Green-yellow} \) |
| \(\text{B.}\) | \( \text{Red} \) | \( \text{Blue-green} \) |
| \(\text{C.}\) | \( \text{Purple} \) | \( \text{Blue-green} \) |
| \(\text{D.}\) | \( \text{Purple} \) | \( \text{Green-yellow} \) |
CHEMISTRY, M7 2023 HSC 10 MC
Which of the following correctly lists the compounds in order of increasing boiling point?
- Heptane < heptan-2-one < heptan-1-o1 < heptanoic acid
- Heptane < heptan-1-o1 < heptan-2-one < heptanoic acid
- Heptanoic acid < heptan-2-one < heptan-1-o1 < heptane
- Heptanoic acid < heptan-1-o1 < heptan-2-one < heptane
CHEMISTRY, M6 2023 HSC 9 MC
A titration was performed using two solutions of equal concentration, producing the following titration curve.
Which combination of solutions does the titration curve represent?
- Addition of a weak base to a weak acid
- Addition of a weak base to a strong acid
- Addition of a strong acid to a weak base
- Addition of a strong acid to a strong base
CHEMISTRY, M5 2023 HSC 7 MC
A mixture of 0.8 mol of \( \ce{CO} \text{(g)} \) and 0.8 mol of \( \ce{H2} \text{(g)} \) was placed in a sealed 1.0 L container. The following reaction occurred.
\( \ce{CO} \text{(g)} + 2 \ce{H2} \text{(g)} \rightleftharpoons \ce{CH3} \ce{OH}\text{(g)} \)
When equilibrium was established, the mixture contained 0.5 mol of \( \ce{CO} \text{(g)} \).
What amount of \( \ce{H2} \text{(g)} \) was present at equilibrium?
- 0.2 mol
- 0.4 mol
- 0.6 mol
- 1.0 mol
CHEMISTRY, M6 2023 HSC 5 MC
PHYSICS, M4 2013 HSC 14 MC
PHYSICS, M3 2018 HSC 6 MC
The diagram shows a saucepan of water on an induction cooktop.
Which row of the table correctly identifies a property of the material used to make the saucepan and the frequency of the changing magnetic field produced by the coil?
| Property of saucepan | Frequency | |
| A. | Insulator | High (50 kHz) |
| B. | Conductor | High (50 kHz) |
| C. | Insulator | Low (50 Hz) |
| D. | Conductor | Low (50 Hz) |
CHEMISTRY, M8 2023 HSC 36
An organic reaction pathway involving compounds \(\text{A, B,}\) and \(\text{C}\) is shown in the flow chart.
The molar mass of \(\text{A}\) is 84.156 g mol\(^{-1}\).
A chemist obtained some spectral data for the compounds as shown.
| \( \text{Data from} \ ^{1} \text{H NMR spectrum of compound C} \) | ||
| \( Chemical \ Shift \ \text{(ppm)} \) | \( Relative \ peak \ area \) | \( Splitting \ pattern \) |
| \(1.01\) | \(3\) | \(\text{Triplet}\) |
| \(1.05\) | \(3\) | \(\text{Triplet}\) |
| \(1.65\) | \(2\) | \(\text{Multiplet}\) |
| \(2.42\) | \(2\) | \(\text{Triplet}\) |
| \(2.46\) | \(2\) | \(\text{Quartet}\) |
| \( ^{1} \text{H NMR chemical shift data}\) | |
| \( Type \ of \ proton \) | \( \text{δ/ppm} \) |
| \( \ce{R - C\textbf{H}3,R - C\textbf{H}2 - R}\) | \(0.7-1.7\) |
| \( \left.\begin{array}{l}\ce{\textbf{H}3C - CO - \\-C\textbf{H}2 - CO -}\end{array}\right\} \begin{aligned} & \text { (aldehydes, ketones,} \\ &\text{carboxylic acids or esters) }\end{aligned}\) | \(2.0-2.6\) |
| \( \ce{R - C\textbf{H}O} \) | \(9.4-10.00\) |
| \( \ce{R - COO\textbf{H}} \) | \(9.0-13.0\) |
Identify the functional group present in each of compounds \(\text{A}\) to \(\text{C}\) and draw the structure of each compound. Justify your answer with reference to the information provided. (9 marks)
--- 28 WORK AREA LINES (style=lined) ---
CHEMISTRY, M8 2023 HSC 2 MC
The technique illustrated is used to analyse chemical substances in a sample.
What is the technique shown?
- Flame test
- Mass spectrometry
- Atomic absorption spectroscopy
- Ultraviolet-visible spectrophotometry
CHEMISTRY, M5 2023 HSC 37
When performing industrial reductions with \(\mathrm{CO}(\mathrm{g})\), the following equilibrium is of great importance.
\( \ce{2CO(g) \rightleftharpoons CO2(g) + C(s) \quad \quad $K$_{e q} = 10.00 at 1095 K } \)
A 1.00 L sealed vessel at a temperature of 1095 K contains \( \ce{CO(g)} \) at a concentration of 1.10 × 10\(^{-2}\) mol L\(^{-1}\), \(\ce{CO2(g)} \) at a concentration of 1.21 × 10\(^{-3}\) mol L\(^{-1}\), and excess solid carbon.
- Is the system at equilibrium? Support your answer with calculations. (2 marks)
--- 3 WORK AREA LINES (style=lined) ---
-
Carbon dioxide gas is added to the system above and the mixture comes to equilibrium. The equilibrium concentrations of \( \ce{CO(g)}\) and \(\ce{CO2(g)} \) are equal. Excess solid carbon is present and the temperature remains at 1095 K.
Calculate the amount (in mol) of carbon dioxide added to the system. (3 marks)
--- 14 WORK AREA LINES (style=lined) ---
CHEMISTRY, M5 2023 HSC 33
Gases \( \ce{A_2} \) and \( \ce{B_2} \) are placed in a closed container of variable volume, as shown.
The reaction between these substances is as follows.
\( \ce{A2(g) + 2B_2(g) \rightleftharpoons 2AB_2(g) \quad \Delta \textit{H} = -10 \text{kJ mol}^{-1}} \)
The following graph shows changes in the amounts (in mol) of these three substances over time in this container.
- Explain what is happening in this system between 6 minutes and 8 minutes. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
- Explain TWO different factors that could result in the disturbance at 8 minutes. (4 marks)
--- 8 WORK AREA LINES (style=lined) ---
CHEMISTRY, M5 2023 HSC 31
Copper(\(\text{II}\)) ions \( \ce{(Cu^{2+})} \) form a complex with lactic acid \( \ce{(C3H6O3)} \), as shown in the equation.
\( \ce{Cu^{2+}(aq)} + \ce{2C3H6O3(aq)} \rightleftharpoons \Bigl[\ce{Cu(C3H6O3)2\Bigr]^{2+}(aq)} \)
This complex can be detected by measuring its absorbance at 730 nm. A series of solutions containing known concentrations of \( \Bigl[\ce{Cu(C3H6O3)_2\Big]^{2+}} \) were prepared, and their absorbances measured.
| \( Concentration \ of \Bigl[\ce{Cu(C3H6O3)_2\Bigr]^{2+}} \) \( \text{(mol L}^{-1}) \) | \( Absorbance \) |
| 0.000 | 0.00 |
| 0.010 | 0.13 |
| 0.020 | 0.28 |
| 0.030 | 0.43 |
| 0.040 | 0.57 |
| 0.050 | 0.72 |
Two solutions containing \( \ce{Cu^{2+}} \ \text{and} \ \ce{C3H6O3} \) were mixed. The initial concentrations of each in the resulting solution are shown in the table.
| \( Species \) | \( Initial \ Concentration\) \( (\text{mol L}^{-1}) \) |
| \( \ce{Cu^{2+}} \) | 0.056 |
| \( \ce{C3H6O3} \) | 0.111 |
When the solution reached equilibrium, its absorbance at 730 nm was 0.66.
You may assume that under the conditions of this experiment, the only species present in the solution are those present in the equation above, and that \( \Bigl[ \ce{Cu(C3H6O3)_2\Bigr]^{2+}} \) is the only species that absorbs at 730 nm.
With the support of a line graph, calculate the equilibrium constant for the reaction. (7 marks)
--- 14 WORK AREA LINES (style=lined) ---
CHEMISTRY, M7 2023 HSC 29
CHEMISTRY, M8 2023 HSC 28
Alkene \(\ce{Q}\) undergoes an addition reaction with chlorine gas to form compound \(\ce{R}\).
- Describe a chemical test that could be done in a school laboratory to confirm that \(\ce{Q}\) is an alkene. Include expected observations in your answer. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
- Compound \(\ce{R}\) was analysed and found to contain approximately 32% carbon by mass. The mass spectrum of compound \(\ce{R}\) is shown.
- Provide a structural formula for compound \(\ce{R}\). Support your answer with calculations. (3 marks)
--- 9 WORK AREA LINES (style=lined) ---
CHEMISTRY, M7 2023 HSC 27
A student has been asked to produce 185 mL of ethanol (MM = 46.068 g mol\(^{-1} \)) by fermenting glucose using yeast, as shown in the equation.
\( \ce{C6H12O6(aq)} \rightarrow \ce{2C2H5OH(aq)} + \ce{2CO2(g)} \)
Given that the density of ethanol is 0.789 g mL\(^{-1} \), calculate the volume of carbon dioxide gas produced at 310 K and 100 kPa. (4 marks)
--- 10 WORK AREA LINES (style=lined) ---
CHEMISTRY, M7 2023 HSC 25
A student used the apparatus shown to investigate the combustion of octan-1-ol.
The following results were obtained by the student.
| Mass of water heated | = 205 g |
| Initial temperature of water | = 23.7°C |
| Final temperature of water | = 60.4°C |
The following data are given.
| Molar enthalpy of combustion of octan-1-ol | = – 5294 kJ mol\(^{-1} \) |
| Molar mass of octan-1-ol | = 130.23 g kJ mol\(^{-1}\) |
- Assuming that no energy released by this combustion is lost to the surroundings, calculate the mass of octan-1-ol burnt. (3 marks)
--- 8 WORK AREA LINES (style=lined) ---
- Explain ONE advantage of using a biofuel compared to fossil fuels. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
CHEMISTRY, M6 2023 HSC 24
The hydrogen oxalate ion \( \ce{(HC2O4^{-})} \) is classified as amphiprotic.
Describe, using chemical equations, how this ion is amphiprotic. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
CHEMISTRY, M6 2023 HSC 23
The pH of two solutions, \(\text{X}\) and \(\text{Y}\), were measured before and after 10 drops of concentrated \( \ce{NaOH} \) was added to each.
Explain the pH changes that occurred in solutions \(\text{X}\) and \(\text{Y}\). (3 marks)
--- 8 WORK AREA LINES (style=lined) ---
CHEMISTRY, M6 2023 HSC 22
Explain how the following substances would be classified under the Arrhenius and Brønsted-Lowry definitions of acids. Support your answer with relevant equations. (4 marks)
-
- \( \ce{HCl(aq)} \)
- \( \ce{NH4Cl(aq)} \)
--- 12 WORK AREA LINES (style=lined) ---
CHEMISTRY, M6 2023 HSC 6 MC
The pH of a solution changes from 8 to 5.
What happens to the concentration of hydrogen ions during this change of pH?
- It increases by a factor of 3.
- It decreases by a factor of 3.
- It increases by a factor of 1000.
- It decreases by a factor of 1000.
BIOLOGY, M6 2023 HSC 35
5-Bromouracil (bU) is a synthetic chemical mutagen. It bonds with adenine in place of thymine in DNA. During replication, it then binds with guanine. This will then make a guanine-cytosine pair on one strand of DNA instead of an adenine-thymine pair. --- 2 WORK AREA LINES (style=lined) --- --- 8 WORK AREA LINES (style=lined) ---
BIOLOGY, M6 2023 HSC 34
Cattle have been domesticated by humans for approximately 10 000 years. Many biotechnologies have been employed in the farming of cattle. The table shows examples of the application of these biotechnologies. \begin{array} {|l|l|} With reference to the table, evaluate the effect of biotechnologies on the biodiversity of cattle. (5 marks)
\hline
\rule{0pt}{2.5ex} \textbf{Biotechnology} \rule[-1ex]{0pt}{0pt} & \textbf{Example} \\
\hline
\rule{0pt}{2.5ex} \text{Selective breeding} & \text{The offspring of highest milk producing female cows were} \\
\text{} & \text{retained and over time cows that produced more milk were bred,} \\
\text{} \rule[-1ex]{0pt}{0pt} & \text{leading to dairy breeds.} \\
\hline
\rule{0pt}{2.5ex} \text{Artificial} & \text{An American bull holds the current record for artificial} \\
\text{insemination} & \text{insemination. He produced 2.4 million units of semen and has} \\
\text{} \rule[-1ex]{0pt}{0pt} & \text{sired cattle in 50 countries.} \\
\hline
\rule{0pt}{2.5ex} \text{Whole organism} & \text{The success rate of cloning cattle is low. There are currently 30-40} \\
\text{cloning} \rule[-1ex]{0pt}{0pt} & \text{cloned cattle in Australia. They are not used commercially.} \\
\hline
\rule{0pt}{2.5ex} \text{Hybridisation } & \text{There are two species of domestic cattle, Bos taurus and Bos} \\
\text{} & \text{indicus. They can be hybridised to breed cattle with} \\
\text{} \rule[-1ex]{0pt}{0pt} & \text{characteristics of both species.} \\
\hline
\rule{0pt}{2.5ex} \text{Transgenic} & \text{The first transgenic cow produced human serum albumin in its} \\
\text{organisms} \rule[-1ex]{0pt}{0pt}& \text{milk. The use of transgenic cattle is not widespread.} \\
\hline
\end{array}
BIOLOGY, M6 2023 HSC 32
The mountain pygmy possum (Burramys parvus) is restricted to four regions in Australia's alpine zone. The species is listed as critically endangered with less than 2000 adults remaining. The range of the mountain pygmy possum has contracted due to a gradually warming climate. Loss and degradation of these habitats have affected local populations. The graph shows changes in the Mt Buller population following recent bushfires and the introduction of male pygmy possums from Mt Bogong. Evaluate how bushfires and the introduction of males from other locations have affected the population size and gene pool of the Mt Buller pygmy possum population. (7 marks) --- 18 WORK AREA LINES (style=lined) ---
BIOLOGY, M7 2023 HSC 30
Tetanus vaccines were introduced in 1953 resulting in reduced case numbers. The majority of recorded cases occurred in people aged 65 and over. The graph shows the vaccination schedule for tetanus. Assess the use of vaccinations and the vaccination schedule. Use the data provided to support your answer. (5 marks)
BIOLOGY, M8 2023 HSC 29b
Organisms use various mechanisms to maintain their internal environment within tolerance limits. Explain TWO adaptations in plants that help to maintain water balance. (4 marks) --- 8 WORK AREA LINES (style=lined) ---
BIOLOGY, M8 2023 HSC 29a
Organisms use various mechanisms to maintain their internal environment within tolerance limits. Outline a physiological adaptation in endotherms which assists in maintaining their internal environment. (2 marks)
BIOLOGY, M7 2023 HSC 28
--- 5 WORK AREA LINES (style=lined) --- --- 5 WORK AREA LINES (style=lined) ---
BIOLOGY, M8 2023 HSC 27
Air pollution has been linked to a variety of non-infectious neurological (brain) disorders. Some of the symptoms include memory loss, cognitive decline and impaired movement and coordination. 500 people from each of three major cities were surveyed and were monitored and tested for a period of 12 months. Each group included males and females aged between 20 and 50 years of age. The results after 12 months were as follows: Evaluate the method used in this epidemiological study in determining a link between air pollution and the symptoms. (7 marks) --- 16 WORK AREA LINES (style=lined) ---
BIOLOGY, M7 2023 HSC 26
Malaria is a potentially fatal infectious disease that is spread to humans by infected mosquitoes. Scientists investigated the behaviour of 20 mosquitoes for an hour in each of the four containers shown. Aim: To determine if wearing clean clothing reduces the transmission of malaria. Assume infected mosquitoes that land on clothing transmit malaria. --- 3 WORK AREA LINES (style=lined) --- --- 6 WORK AREA LINES (style=lined) ---
\begin{array}{|c|c|c|c|c|}
\hline
\rule{0pt}{2.5ex} \textit {Experiment} \rule[-1ex]{0pt}{0pt}& \textit {Container A} & \textit {Container B} & \textit {Container C} & \textit {Container D} \\
\hline
\rule{0pt}{2.5ex} 1 \rule[-1ex]{0pt}{0pt}& 15 & 7 & 12 & 5 \\
\hline
\rule{0pt}{2.5ex} 2 \rule[-1ex]{0pt}{0pt}& 19 & 5 & 9 & 3 \\
\hline
\rule{0pt}{2.5ex} 3 \rule[-1ex]{0pt}{0pt}& 12 & 4 & 14 & 6 \\
\hline
\rule{0pt}{2.5ex} 4 \rule[-1ex]{0pt}{0pt}& 18 & 6 & 13 & 4 \\
\hline
\rule{0pt}{2.5ex} 5 \rule[-1ex]{0pt}{0pt}& 19 & 6 & 10 & 3 \\
\hline
\end{array}
BIOLOGY, M5 2023 HSC 25d
The normal Huntingtin protein has 10−26 repeats of CAG. In Huntington’s 2 disease there are 37−80 repeats.
Diagram 1 shows a pedigree of a family known to be affected by Huntington's disease. Diagram 2 shows the results of gel electrophoresis on fragments of DNA from chromosome four, known to be altered in Huntington's disease.
Diagram 1
Diagram 2
Predict whether individuals \(S\) and \(U\) will be affected by Huntington's disease, and if so, at what age. Use data from the diagrams to justify your answer. (3 marks)
--- 6 WORK AREA LINES (style=lined) ---
BIOLOGY, M5 2023 HSC 25b
Huntington's disease is caused by a misfolded protein 'Huntingtin'. It is caused by excess repeats of the DNA sequence CAG on the coding strand of DNA. The mRNA that is produced has the same sequence as the DNA. Use the codon chart, starting in the centre, to identify the amino acid that is repeated. (1 mark)
BIOLOGY, M5 2023 HSC 25a
BIOLOGY, M8 2023 HSC 24
--- 6 WORK AREA LINES (style=lined) --- --- 6 WORK AREA LINES (style=lined) ---
PHYSICS, M8 2023 HSC 27a
Explain how the composition and temperature of a star can be determined from its spectrum. (4 marks) --- 10 WORK AREA LINES (style=lined) ---
PHYSICS, M8 2023 HSC 26
Consider the following nuclear reaction \({ }_{\ \ 6}^{12} \text{C} +{ }_1^1 \text{H} \rightarrow{ }_5^9 \text{B} +{ }_2^4 \text{He}\) The masses of the isotopes in this process are shown in the table. --- 6 WORK AREA LINES (style=lined) ---
Isotope
Mass (\(u\))
\({ }_{\ \ 6}^{12} \text{C}\)
12.064
\({ }_5^9 \text{B}\)
9.013
\({ }_2^4 \text{He}\)
4.003
\({ }_1^1 \text{H}\)
1.008
PHYSICS, M6 2023 HSC 24
PHYSICS, M7 2023 HSC 23b
The James Webb Space Telescope (JWST) is sensitive to wavelengths from 6.0 \(\times\) 10\(^{-7}\) m to 2.8 \(\times\) 10\(^{-5}\) m. What is the minimum photon energy that it can detect? (3 marks) --- 6 WORK AREA LINES (style=lined) ---
PHYSICS, M8 2023 HSC 21
PHYSICS, M5 2023 HSC 14 MC
Planet \(X\) has a mass 4 times that of Earth and a radius 3 times that of Earth. The escape velocity at the surface of Earth is 11.2 km s\(^{-1}\).
What is the escape velocity at the surface of planet \(X\) ?
- 8.40 km s\(^{-1}\)
- 9.70 km s\(^{-1}\)
- 12.9 km s\(^{-1}\)
- 14.9 km s\(^{-1}\)
PHYSICS, M7 2023 HSC 9 MC
The graph shows the relationship between radiation intensity and wavelength for a black body at 4500 K.
Which statement describes the expected difference in the graph for a black body at 4000 K?
- Intensity at all wavelengths will be less.
- Intensity at all wavelengths will be greater.
- The peak intensity will occur at a higher frequency.
- The peak intensity will occur at a shorter wavelength.
PHYSICS, M5 2023 HSC 8 MC
PHYSICS, M8 2023 HSC 7 MC
A proton and a neutron travel at the same speed.
Which statement correctly explains the difference between their de Broglie wavelengths?
- The proton has a longer wavelength because its mass is greater.
- The proton has a shorter wavelength because its mass is smaller.
- The neutron has a shorter wavelength because its mass is greater.
- The neutron has a longer wavelength because its mass is smaller.
PHYSICS, M5 2023 HSC 5 MC
PHYSICS, M7 2023 HSC 3 MC
BIOLOGY, M4 EQ-Bank 24
Explain ONE example of a mutual symbiotic relationship. (3 marks)
BIOLOGY, M4 EQ-Bank 7 MC
A tapeworm is an animal that gains it's nutrients while living inside the host, often making the host seriously ill.
What kind of symbiotic relationship is this an example of?
- Predation.
- Parasitism.
- Mutualism.
- Commensalism.
BIOLOGY, M5 2023 HSC 23
The following graph outlines some hormonal changes during pregnancy.
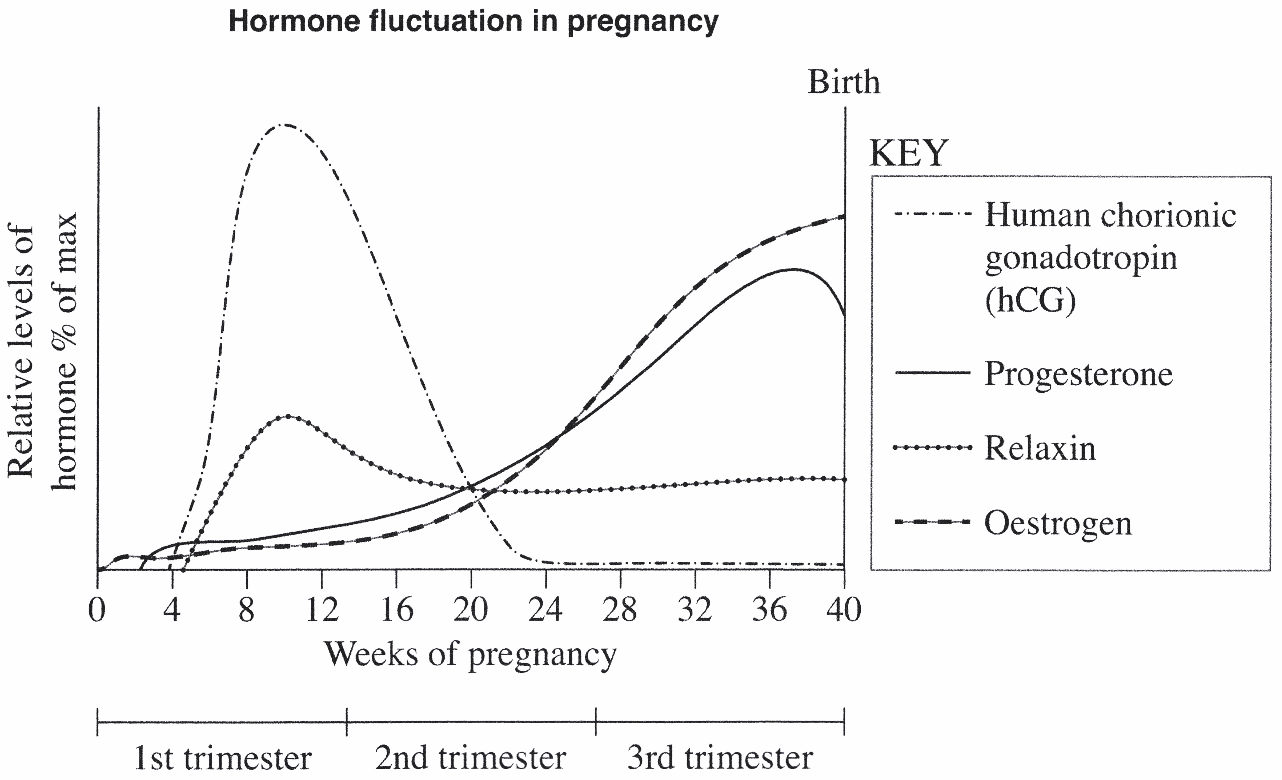
Complete the table for TWO of the hormones graphed. (4 marks)
\begin{array} {|c|c|c|}
\hline
\rule{0pt}{2.5ex} \quad \textit{Hormone name} \quad \rule[-1ex]{0pt}{0pt} & \quad \quad \textit{Function in pregnancy} \quad \quad & \quad \textit{Trimester where}\quad \\
\text{} \rule[-1.5ex]{0pt}{0pt} & \text{} & \textit{peak occurs}\\
\hline
\text{} & \text{} & \text{} \\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{} \\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\hline
\text{} & \text{} & \text{} \\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{} \\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\text{} & \text{} & \text{}\\
\hline
\end{array}
--- 0 WORK AREA LINES (style=lined) ---
BIOLOGY, M7 2023 HSC 22a
Describe how phagocytes help protect against pathogens. (2 marks)
BIOLOGY, M5 2023 HSC 21a
Identify the components of a nucleotide. (1 mark)
BIOLOGY, M6 2023 HSC 20 MC
The diagram shows the karyotype of a normal female Tasmanian devil cell and the karyotype of a Tasmanian devil facial tumour cell.
M1, M2, M3 and M4 are marker chromosomes. These are chromosomes of unknown origin additional to the normal chromosomes found in the cells of Tasmanian devil facial tumour disease.
What can be deduced from the karyotypes?
- The karyotype of the tumour cells shows trisomy.
- The karyotype of tumour cells contains multiple chromosomal inversions.
- The karyotype of tumour cells contains both chromosomal insertions and deletions.
- The karyotype of the tumour cells contains more chromosomes than the karyotype of the normal Tasmanian devil cells.
BIOLOGY, M6 2023 HSC 19 MC
The following are the five steps in the process of gene cloning.
- Selection of organisms containing recombinant DNA sequences
- Creation of recombinant DNA joined using DNA ligase
- Introduction of recombinant DNA into host organism
- Extraction and amplification of DNA to be cloned
- Choice of host organism and cloning vector
Which is the correct order for this process?
- 5, 2, 1, 3, 4
- 5, 4, 2, 3, 1
- 3, 2, 4, 5, 1
- 4, 5, 1, 2, 3
- « Previous Page
- 1
- …
- 43
- 44
- 45
- 46
- 47
- …
- 114
- Next Page »