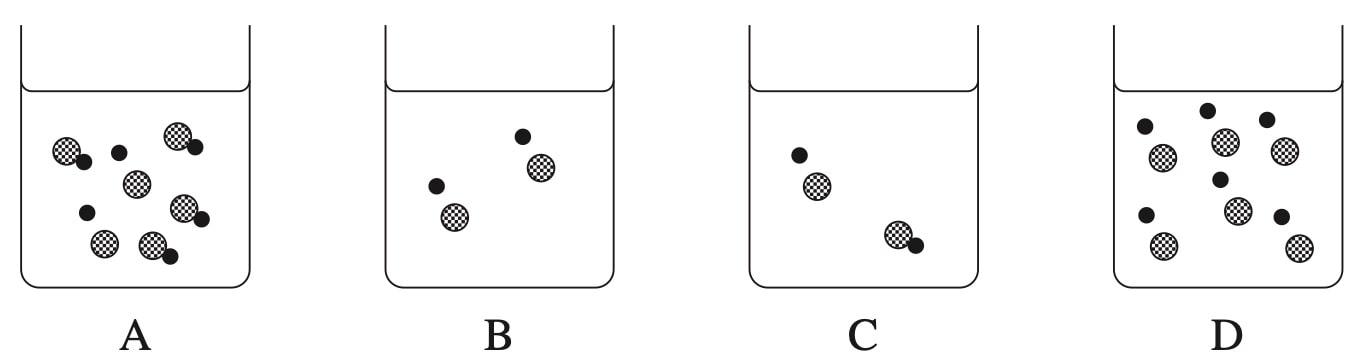
A controlled experiment was performed to investigate the effect of substrate concentration on the rate of an enzyme-catalysed reaction. Data were collected and are presented in the graph.
- What is the independent variable in this experiment? (1 mark)
--- 1 WORK AREA LINES (style=lined) ---
- Explain the trends shown in the graph. (3 marks)
--- 6 WORK AREA LINES (style=lined) ---