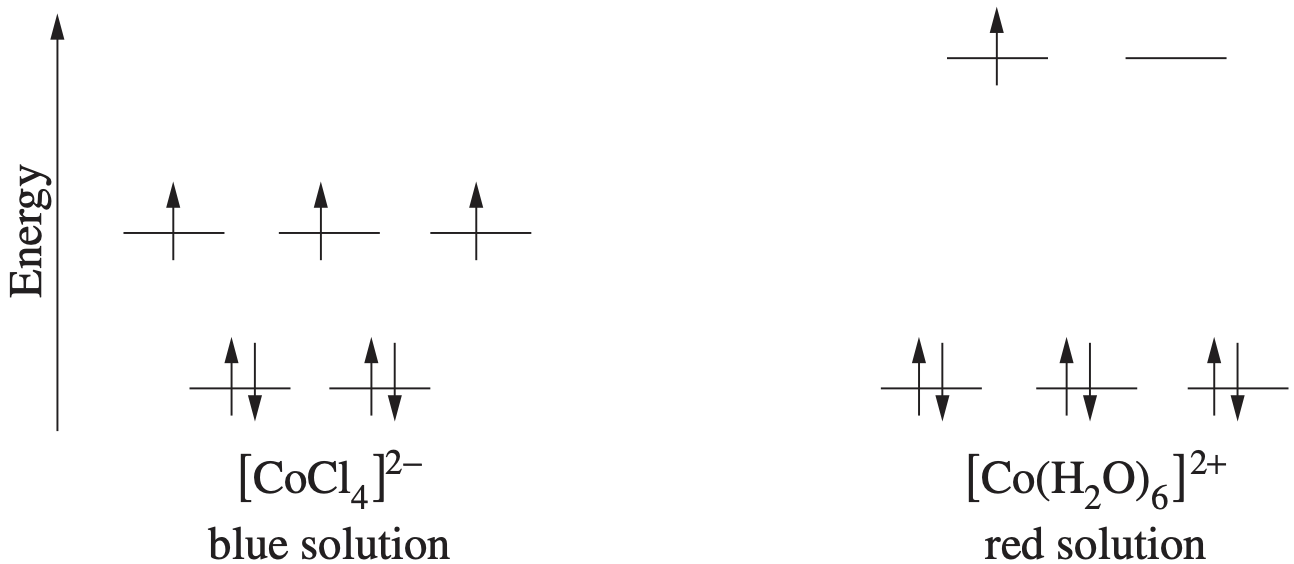
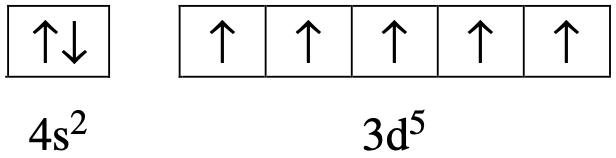
The nitrogen content of bread was determined using the following procedure:
-
- A sample of bread weighing 2.80 g was analysed.
- The nitrogen in the sample was converted into ammonia.
- The ammonia was collected in 50.0 mL of 0.125 mol L\(^{-1}\) hydrochloric acid. All of the ammonia was neutralised, leaving an excess of hydrochloric acid.
- The excess hydrochloric acid was titrated with 23.30 mL of 0.116 mol L\(^{-1}\) sodium hydroxide solution.
- Write balanced equations for the TWO reactions involving hydrochloric acid. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
- Calculate the moles of excess hydrochloric acid. (1 mark)
--- 2 WORK AREA LINES (style=lined) ---
- Calculate the moles of ammonia. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---
- Calculate the percentage by mass of nitrogen in the bread. (2 marks)
--- 4 WORK AREA LINES (style=lined) ---