A pathogen and a red blood cell are drawn to the same scale, with some features indicated.
What type of pathogen is this?
- A virus
- A prion
- A fungus
- A bacterium
Aussie Maths & Science Teachers: Save your time with SmarterEd
A pathogen and a red blood cell are drawn to the same scale, with some features indicated.
What type of pathogen is this?
`C`
`=>C`
The boiling points and molar masses of three compounds are shown in the table.
Acetic acid, butan-1-ol and butyl acetate have very different molar masses but similar boiling points. Explain why in terms of the structure and bonding of the three compounds. (5 marks)
The following reaction scheme can be used to synthesise ethyl ethanoate.
Outline the reagents and conditions required for each step and how the product of each step could be identified. (7 marks)
--- 16 WORK AREA LINES (style=lined) ---
Step 1:
Step 2:
Step 3
Step 1:
Step 2:
Step 3
The following data apply to magnesium fluoride and magnesium chloride dissolving in water at 298 K.
\begin{array}{|l|c|c|}
\hline
\rule{0pt}{2.5ex} & \textit{Magnesium fluoride} \rule[-1ex]{0pt}{0pt}& \textit{Magnesium chloride}\\
\hline
\rule{0pt}{2.5ex}\Delta_{sol} H^{\ominus}\left(\text{kJ mol}^{-1}\right) \rule[-1ex]{0pt}{0pt}& -7.81 & -160 \\
\hline
\rule{0pt}{2.5ex}\Delta_{\text {sol }} S^{\ominus}\left(\text{J K}^{-1} mol^{-1}\right) \rule[-1ex]{0pt}{0pt}& -223 & -115 \\
\hline
\rule{0pt}{2.5ex}T \Delta_{\text {sol }} S^{\ominus}\left(\text{kJ mol}^{-1}\right) \rule[-1ex]{0pt}{0pt}& -66.4 & -34.2 \\
\hline
\rule{0pt}{2.5ex}\Delta_{\text {sol }} G^{\ominus}\left(\text{kJ mol}^{-1}\right) \rule[-1ex]{0pt}{0pt}& +58.6 & -125 \\
\hline
\end{array}
Compare the effects of enthalpy and entropy on the solubility of these salts. (3 marks)
--- 8 WORK AREA LINES (style=lined) ---
Stormwater from a mine site has been found to be contaminated with copper\(\text{(II)}\) and lead\(\text{(II)}\) ions. The required discharge limit is 1.0 mg L¯1 for each metal ion. Treatment of the stormwater with \(\ce{Ca(OH)2}\) solid to remove the metal ions is recommended.
--- 5 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
--- 7 WORK AREA LINES (style=lined) ---
a. Recommended Treatment:
b. Atomic absorption spectroscopy (AAS):
c. Concentrations of ions:
\begin{array} {|l|c|c|c|}
\hline \text{Sample }& \ce{Cu^2+ \times 10^{-5} mol L^{-1}} & \ce{Pb^2+ \times 10^{-5} mol L^{-1}} \\
\hline \text{Water (pre-treatment)} & 5.95 & 4.75 \\
\hline \text{Water (post-treatment)} & 0.25 & 0.85 \\
\hline \end{array}
\begin{array} {ccc}
\ce{Cu^2+}: & 5.95 \times 10^{-5} \times 63.55 \times 1000 = 3.78\ \text{mg L}^{-1} \\
& 0.25 \times 10^{-5} \times 63.55 \times 1000 = 0.16\ \text{mg L}^{-1} \\
& \\
\ce{Pb^2+}: & 4.75 \times 10^{-5} \times 207.2 \times 1000 = 9.84\ \text{mg L}^{-1} \\
& 0.85 \times 10^{-5} \times 207.2 \times 1000 = 1.76\ \text{mg L}^{-1} \end{array}
Conclusion:
a. Recommended Treatment:
b. Atomic absorption spectroscopy (AAS):
c. Concentrations of ions:
\begin{array} {|l|c|c|c|}
\hline \text{Sample }& \ce{Cu^2+ \times 10^{-5} mol L^{-1}} & \ce{Pb^2+ \times 10^{-5} mol L^{-1}} \\
\hline \text{Water (pre-treatment)} & 5.95 & 4.75 \\
\hline \text{Water (post-treatment)} & 0.25 & 0.85 \\
\hline \end{array}
\begin{array} {ccc}
\ce{Cu^2+}: & 5.95 \times 10^{-5} \times 63.55 \times 1000 = 3.78\ \text{mg L}^{-1} \\
& 0.25 \times 10^{-5} \times 63.55 \times 1000 = 0.16\ \text{mg L}^{-1} \\
& \\
\ce{Pb^2+}: & 4.75 \times 10^{-5} \times 207.2 \times 1000 = 9.84\ \text{mg L}^{-1} \\
& 0.85 \times 10^{-5} \times 207.2 \times 1000 = 1.76\ \text{mg L}^{-1} \end{array}
Conclusion:
Assess the usefulness of the Brønsted-Lowry model in classifying acids and bases. Support your answer with at least TWO chemical equations. (5 marks)
25.0 mL of a 0.100 mol L ¯1 acid is to be titrated against a sodium hydroxide solution until final equivalence is reached.
Which of the following acids, if used in the titration, would require the greatest volume of sodium hydroxide?
`B`
`=>B`
A part of a cathode ray oscilloscope was represented on a website as shown.
Electrons leave the cathode and are accelerated towards the anode.
--- 7 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. Inaccuracies include:
b. `F=4xx10^(-14) text{N}`
c. `v=4.19 xx10^(7) text{ms}^(-1)`
a. Inaccuracies include:
b. Using `E=(F)/(q)` and `E=(V)/(d):`
| `(F)/(q)` | `=(V)/(d)` | |
| `F` | `=(Vq)/(d)` | |
| `=(5000 xx1.6 xx10^(-19))/(0.02)` | ||
| `=4xx10^(-14) text{N}` |
c. `a=(F)/(m)=(4xx10^(-14))/(9.1 xx10^(-31))=4.4 xx10^(16) text{ms}^(-1)`
| `v^(2)` | `=u^(2)+2as` | |
| `:.v` | `=sqrt(2xx4.4 xx10^(16)xx0.02)` | |
| `=4.19 xx10^(7) text{ms}^(-1)` |
An astronaut working outside a spacecraft in orbit around Earth is not attached to it.
Why does the astronaut NOT drift away from the spacecraft?
`D`
By Elimination:
`=>D`
Which of the following diagrams correctly represents the force(s) acting on a satellite in a stable circular orbit around Earth?
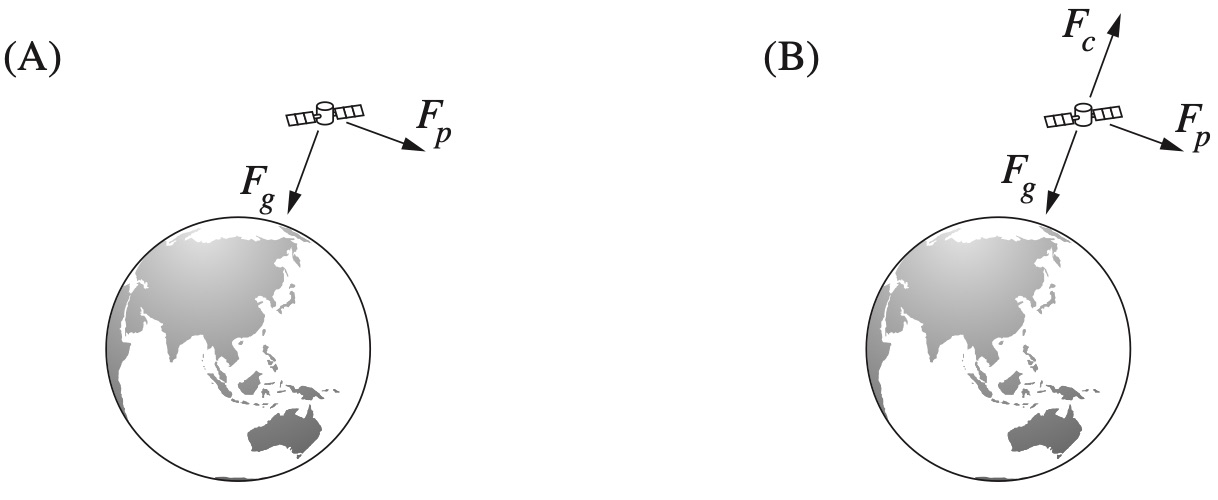
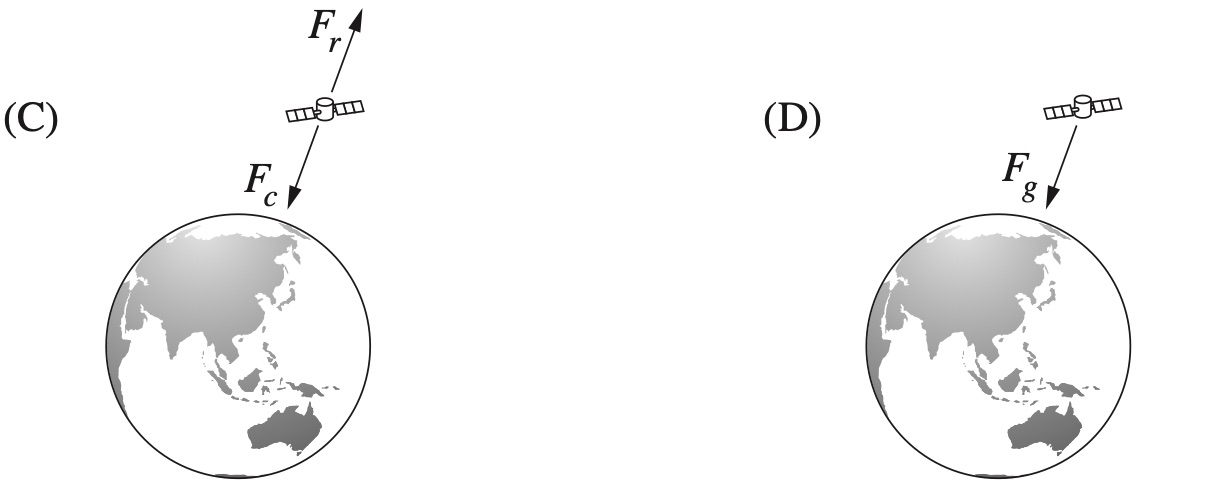
`D`
`=>D`
The following makeshift device was made to provide lighting for a stranded astronaut on Mars.
The mass of Mars is `6.39 ×10^(23) \ text {kg}`.
The 2 kg mass falls, turning the DC generator, which supplies energy to the light bulb. The mass falls from a point that is 3 376 204 m from the centre of Mars.
--- 8 WORK AREA LINES (style=lined) ---
--- 8 WORK AREA LINES (style=lined) ---
a. 7.48 J
b. When switch is opened:
a. `DeltaE=U_(f)-U_(i)`
`=((-Gm_(1)m_(2))/(r_(f)))-((-Gm_(1)m_(2))/(r_(i)))`
`=(-6.67 xx10^(-11)xx6.39 xx10^(23)xx2)/(3\ 376\ 203)-((-6.67 xx10^(-11)xx6.39 xx10^(23)xx2))/(3\ 376\ 204)`
`=-7.48\ text{J}`
b. When switch is opened:
In the motor shown, the rotor spins clockwise, as viewed from point `P`, when connected to a DC supply.
What happens when the motor is connected to an AC supply?
`B`
`=>B`
The cone of a speaker is pushed so that the coil moves in the direction shown.
Which row of the table correctly identifies the behaviour of the speaker and the direction of the current through the conductor?
\begin{align*}
\begin{array}{l}
\rule{0pt}{1.5ex}\textit{} & \textit{} \\
\textit{}\rule[1ex]{0pt}{0pt}& \textit{} \\
\rule{0pt}{2.5ex}\textbf{A.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{B.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{C.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{D.}\rule[-1ex]{0pt}{0pt}\\
\end{array}
\begin{array}{|c|c|}
\hline
\rule{0pt}{1.5ex}\textit{The speaker} & \textit{The direction of the} \\
\textit{behaves like a ...}\rule[1ex]{0pt}{0pt}& \textit{current is from ...} \\
\hline
\rule{0pt}{2.5ex}\text{generator}\rule[-1ex]{0pt}{0pt}&\text{\(X\) to \(Y\)}\\
\hline
\rule{0pt}{2.5ex}\text{generator}\rule[-1ex]{0pt}{0pt}& \text{\(Y\) to \(X\)}\\
\hline
\rule{0pt}{2.5ex}\text{motor}\rule[-1ex]{0pt}{0pt}& \text{\(X\) to \(Y\)} \\
\hline
\rule{0pt}{2.5ex}\text{motor}\rule[-1ex]{0pt}{0pt}& \text{\(Y\) to \(X\)} \\
\hline
\end{array}
\end{align*}
\(A\)
\(\Rightarrow A\)
Contrast the design of transformers and magnetic braking systems in terms of the effects that eddy currents have in these devices. (6 marks)
--- 15 WORK AREA LINES (style=lined) ---
Transformers:
Magnetic Braking Systems:
Transformers:
Magnetic Braking Systems:
The diagram shows an electric circuit in a magnetic field directed into the page. The graph shows how the flux through the conductive loop changes over a period of 12 seconds.
--- 4 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. `2.1\ text{T}`
b. `0.3 text{V}`
| a. | `Phi` | `=BA` |
| `B` | `=(Phi)/(A)=(Phi)/(pir^(2))` | |
| `B_max` | `=(0.6)/(pi xx(0.3)^(2))=2.1\ text{T}` |
b. Voltage (emf) = time rate of flux
Earth's magnetic field is shown in the following diagram.
Two students standing a few metres apart on the equator at points `X` and `Y`, where Earth's magnetic field is parallel to the ground, hold a loop of copper wire between them. Part of the loop is rotated like a skipping rope as shown, while the other part remains motionless on the ground.
At what point during the rotation of the wire does the maximum current flow in a direction from `P` to `Q` through the moving part of the wire?
`C`
`=>C`
A magnet rests on an electronic balance. A rigid copper rod runs horizontally through the magnet, at right angles to the magnetic field. The rod is anchored so that it cannot move.
Which expression can be used to calculate the balance reading when the switch is closed?
`B`
`=>B`
The diagram shows a model of a system used to distribute energy from a power station through transmission lines and transformers to houses.
During the evening peak period there is an increase in the number of electrical appliances being turned on in houses.
Explain the effects of this increased demand on the components of the system, with reference to voltage, current and energy. (6 marks)
--- 18 WORK AREA LINES (style=lined) ---
Current and voltage:
Energy losses:
Current and voltage:
Energy losses:
The diagram shows two right-angled triangles, `A B C` and `A B D`,
where `A C=35 \ text{cm}`, `B D=93 \ text{cm}`, `/_ A C B=41^@` and ` /_ A D B=\theta`.
Calculate the size of angle `\theta`, to the nearest minute. (4 marks)
--- 8 WORK AREA LINES (style=lined) ---
`19^@6`′
`text{In}\ Delta ABC:`
| `tan 41^@` | `=(AB)/35` | |
| `AB` | `=35xxtan 41^@` | |
| `=30.425…` |
`text{In}\ Delta ABD:`
| `sin theta` | `=(AB)/(BD)` | |
| `=(30.425…)/93` | ||
| `:.theta` | `=sin^(-1)((30.425…)/93)` | |
| `=19.09…` | ||
| `=19^@6`′`\ \ text{(nearest minute)}` |
The ages of the 10 members in a tennis club are
`{:[24,25,27,33,34,34,35,39,47,59.]:}`
Could the age `59` be considered an outlier? Justify your answer with calculations. (3 marks)
--- 6 WORK AREA LINES (style=lined) ---
`Q_1=27, \ Q_3=39`
`IQR = Q_3-Q_1 = 39-27 = 12`
`text{Upper limit:}`
| `Q_3 + 1.5 xx IQR` | `=39 + 1.5 xx 12` | |
| `=39 + 1.5 xx 12` | ||
| `=57` |
`:.\ text{S}text{ince 59 > 57, 59 is an outlier.}`
`Q_1=27, \ Q_3=39`
`IQR = Q_3-Q_1 = 39-27 = 12`
`text{Upper limit:}`
| `Q_3 + 1.5 xx IQR` | `=39 + 1.5 xx 12` | |
| `=39 + 1.5 xx 12` | ||
| `=57` |
`:.\ text{S}text{ince 59 > 57, 59 is an outlier.}`
Experiments were conducted to obtain data on the traits 'seed shape' in plants and 'feather colour' in chickens. In each case, the original parents were pure breeding and produced the first generation (F1). The frequency data diagrams below relate to the second generation offspring (F2), produced when the F1 generations were bred together.
Explain the phenotypic ratios of the F2 generation in both the plant and chicken breeding experiments. Include Punnett squares and a key to support your answer. (5 marks)
--- 12 WORK AREA LINES (style=lined) ---
\begin{array} {|c|c|c|}\hline & \text{R} & \text{r} \\ \hline \text{R} & \text{RR} & \text{Rr} \\ \hline \text{r} & \text{Rr} & \text{rr} \\ \hline \end{array}
Key: R = Round r = wrinkled
\begin{array} {|c|c|c|}\hline & \text{B} & \text{W} \\ \hline \text{B} & \text{BB} & \text{BW} \\ \hline \text{W} & \text{BW} & \text{WW} \\ \hline \end{array}
Key: B = Black Feathers W= White Feathers
\begin{array} {|c|c|c|}\hline & \text{R} & \text{r} \\ \hline \text{R} & \text{RR} & \text{Rr} \\ \hline \text{r} & \text{Rr} & \text{rr} \\ \hline \end{array}
Key: R = Round r = wrinkled
\begin{array} {|c|c|c|}\hline & \text{B} & \text{W} \\ \hline \text{B} & \text{BB} & \text{BW} \\ \hline \text{W} & \text{BW} & \text{WW} \\ \hline \end{array}
Key: B = Black Feathers W= White Feathers
Describe ONE mechanism by which plants maintain internal water homeostasis. (3 marks)
--- 8 WORK AREA LINES (style=lined) ---
Huntington's disease is an autosomal dominant condition caused by a mutation of a gene on chromosome 4. It causes nerve cells to break down.
Stargardt disease is an autosomal recessive condition caused by a mutation of a different gene on chromosome 4 . It causes damage to the retina.
A patient is heterozygous for both Huntington's (Hh) and Stargardt disease (Rr). His father's extended family has numerous cases of both of these diseases. His mother does not have either disease and is homozygous for both genes.
--- 0 WORK AREA LINES (style=lined) ---
The map shows the percentage of adult indigenous populations able to digest lactose.
The ability to digest lactose is due to the presence of an enzyme (lactase) which can metabolise the sugar (lactose) present in milk. The gene responsible for producing lactase is usually permanently switched off at some time between the ages of 2 and 5 years. However, some people remain able to digest lactose throughout their lives.
With reference to evolution and DNA, provide possible reasons for the distribution shown in the map. (5 marks)
The diagram shows a container, closed at the base. It is to be filled with water at a constant rate.
Which graph best shows the depth of water in the container as time varies?
`A`
The container will fill at a constant rate for the cylindrical section of the container → a straight line (linear graph).
The container will fill more slowly at a decreasing rate for the conical section of the container → a curved line (non-linear graph).
Therefore, `A` and `B` are the only options.
It cannot be graph `B` as it shows the depth increasing at an increasing rate after the straight line section.
`=>A`
Alzheimer's disease causes destruction of brain tissue, dementia and eventually death.
The diagram shows the effect of Alzheimer's disease on the brain.
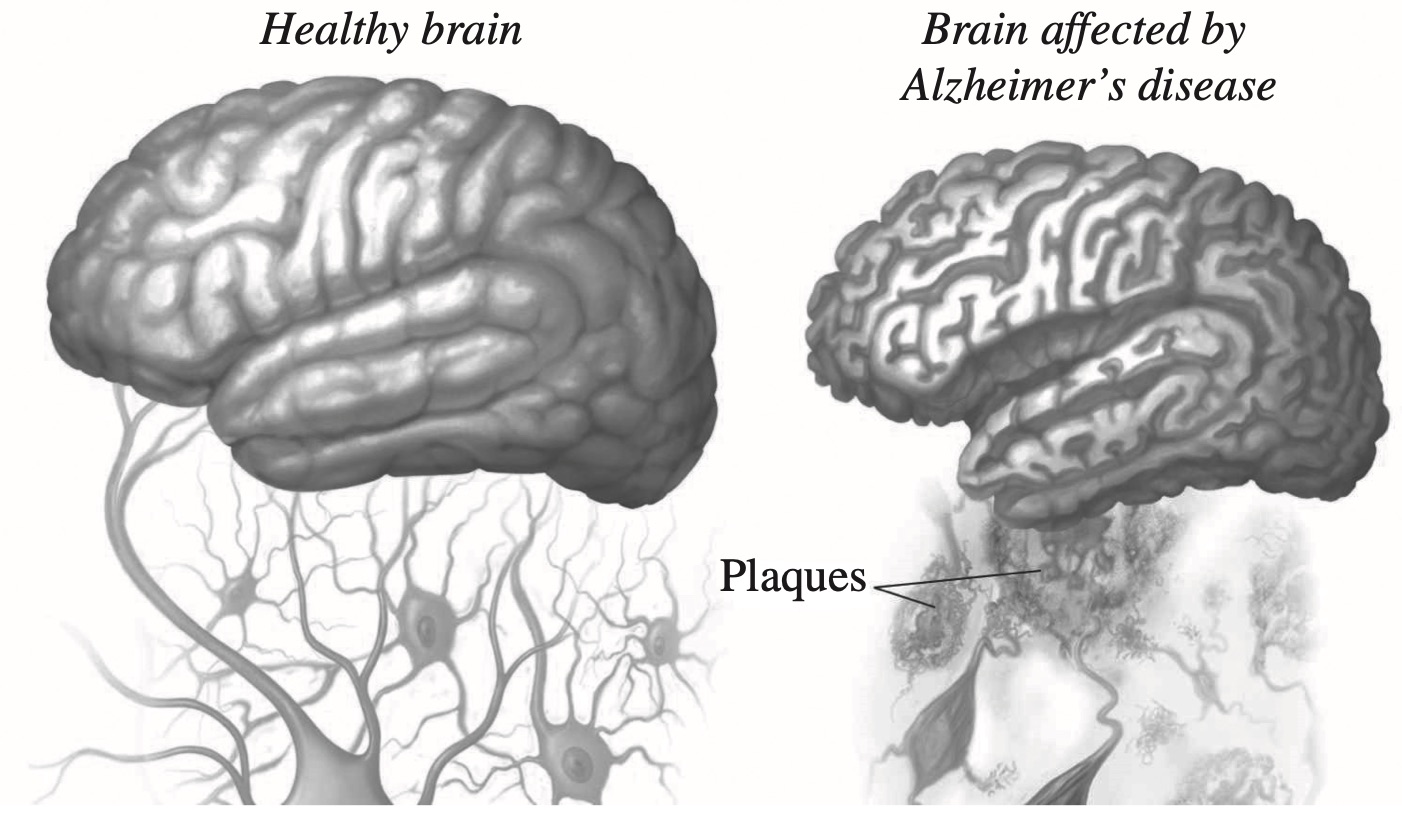
Amyloid beta protein is produced in the human brain throughout life. In people with Alzheimer's disease, it accumulates in excessive amounts.
The gene with the greatest known effect on the risk of developing late-onset Alzheimer's disease is called APOE. It is found on chromosome 19.
The APOE gene has multiple alleles, including e2, e3 and e4 .
The table shows the risk of developing Alzheimer's disease for various APOE genotypes compared to average risk in the population.
A large epidemiological study was conducted. It used historical data to investigate the association between Herpes simplex virus (HSV) infection and dementia. Dementia is caused by a variety of brain illnesses. Alzheimer's disease is the most common cause of dementia.
The study used the records of 8362 patients with HSV infection and 25086 randomly selected sex- and age-matched control patients without HSV infection. Some of the patients with HSV had been treated with antiviral medication.
The graph below shows some results of the study.
Diseases are classified as infectious or non-infectious.
Evaluate whether Alzheimer's disease should be classified as an infectious disease or a non-infectious disease. In your answer, include reference to the information and data provided above. (8 marks)
--- 18 WORK AREA LINES (style=lined) ---
Infectious vs non-infection disease classification
Conclusion
Infectious vs non-infection disease classification
Conclusion
A human karyotype that shows evidence of chromosomal mutation is shown.
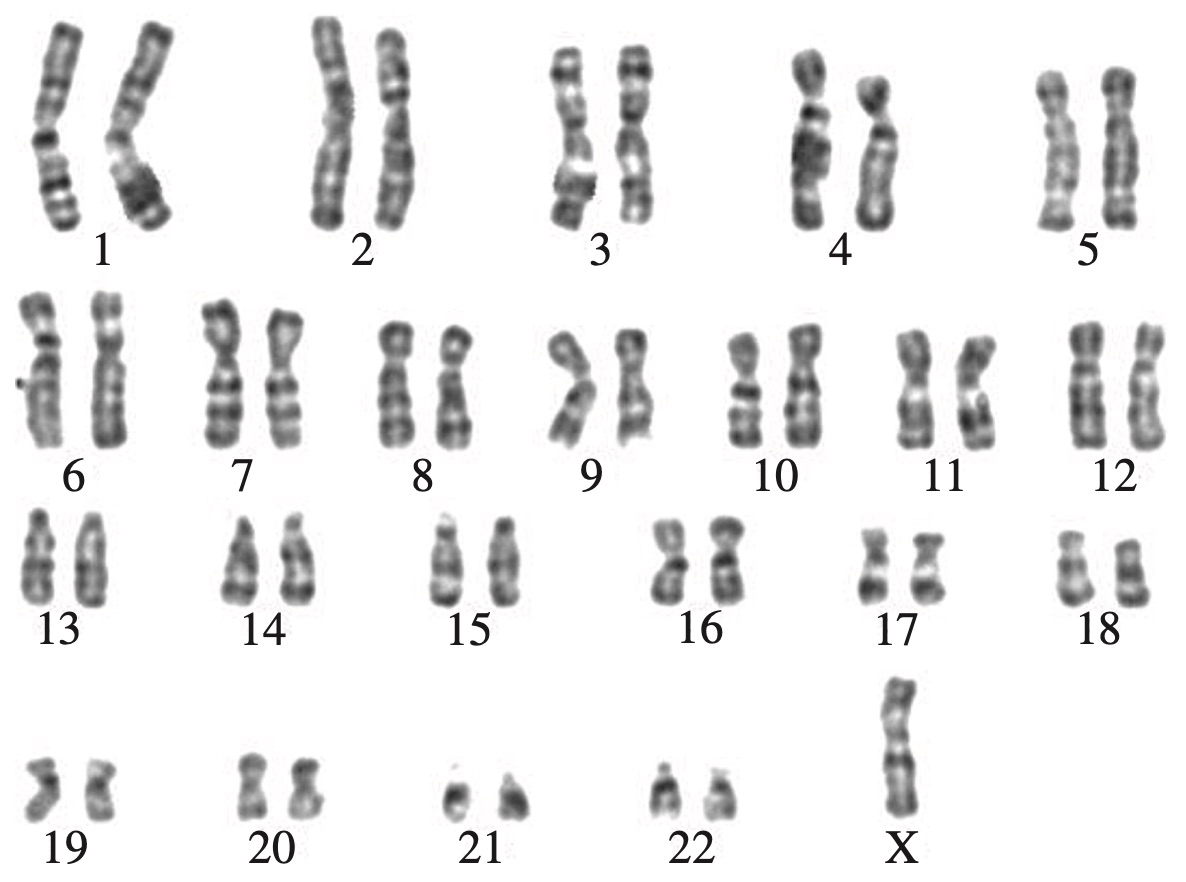
--- 1 WORK AREA LINES (style=lined) ---
--- 8 WORK AREA LINES (style=lined) ---
a. There is only one copy of the sex chromosome (Monosomy, Turner Syndrome).
b. Process of producing karyotype:
a. There is only one copy of the sex chromosome (Monosomy, Turner Syndrome).
b. Process of producing karyotype:
Let \(A\) and \(B\) be two distinct points in three-dimensional space. Let \(M\) be the midpoint of \(A B\).
Let \(S_1\) be the set of all points \(P\) such that \(\overrightarrow{AP} \cdot \overrightarrow{BP}=0\).
Let \(S_2\) be the set of all points \(N\) such that \(\Big|\overrightarrow{AN}\Big|=\Big| \overrightarrow{MN} \Big| \).
The intersection of \(S_1\) and \(S_2\) is the circle \(S\).
What is the radius of the circle \(S\) ?
\(D\)
`text{Diagram below is a 2-D sliced image of the 3-D geometry:}`
\(\overrightarrow{AP} \cdot \overrightarrow{BP}=0\).
\(r=\dfrac{\Big| \overrightarrow{AB} \Big|}{2} \)
\(S_2\ \text{includes}\ N\ \text{where}\ \Big| \overrightarrow{AN} \Big|=\Big| \overrightarrow{MN} \Big| = \dfrac{r}{2} \)
\(\text{Let}\ r_s=\ \text{radius of}\ S\)
\(\text{Point}\ P\ \text{is intersection of}\ S_1\ \text{and}\ S_2 \)
\(\text{By Pythagoras (see diagram):}\)
| \(r_s^2\) | \(=r^2-(\dfrac{r}{2})^2 \) | |
| \(=\dfrac{3r^2}{4}\) | ||
| \(r_s\) | \(=\dfrac{\sqrt3}{2} \times r\) | |
| \(=\dfrac{\sqrt3}{2} \cdot \dfrac{\Big| \overrightarrow{AB} \Big|}{2} \) | ||
| \(=\dfrac{\sqrt3 \Big| \overrightarrow{AB} \Big|}{4} \) |
\(=>D\)
Find all the complex numbers `z_1, z_2, z_3` that satisfy the following three conditions simultaneously. (3 marks)
`{[|z_(1)|=|z_(2)|=|z_(3)|],[z_(1)+z_(2)+z_(3)=1],[z_(1)z_(2)z_(3)=1]:}`
--- 8 WORK AREA LINES (style=lined) ---
`z_1,z_2,z_3=1,i,-i \ \ text{(in any order)}`
`z_1z_2z_3=1\ \ =>\ \ abs(z_1) abs(z_2) abs(z_3)=1`
`text{Given}\ \ abs(z_1) = abs(z_2) = abs(z_3)`
`=>abs (z_1)^3=1 \ \ => \ \ abs(z_1)=1`
`:.abs(z_1) = abs(z_2) = abs(z_3)=1`
`text{Consider}\ \ z_1=e^(itheta):`
`1/z_1=e^(-itheta)=bar(e^(itheta))=bar(z)_1`
`text{Similarly,}`
`1/z_2=bar(z)_2, \ 1/z_3=bar(z)_3`
`=>z_1z_2z_3=1`
`text{Consider}\ \ z_1z_2:`
`z_1z_2=1/z_3=barz_3`
`text{Similarly,}`
`z_2z_3=1/z_1=barz_1, \ z_1z_3=1/z_2=barz_2`
| `z_1z_2+z_1z_3+z_2z_3` | `=barz_3+barz_2+barz_1` | |
| `=bar(z_1+z_2+z_3)` | ||
| `=1` |
`z_1, z_2,z_3\ \ text{are zeros of polynomial:}`
| `z^3-z^2+z-1` | `=0` | |
| `(z-1)(z^2+1)` | `=0` |
`z_1,z_2,z_3=1,i,-i \ \ text{(in any order)}`
It is given that for positive numbers `x_(1),x_(2),x_(3),dots,x_(n)` with arithmetic mean `A`,
`(x_(1)xxx_(2)xxx_(3)xx cdots xxx_(n))/(A^(n)) <= 1` (Do NOT prove this.)
Suppose a rectangular prism has dimensions `a,b,c` and surface area `S`.
--- 6 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
i. `text{Show}\ \ abc <= ((S)/(6))^((3)/(2))`
`S=2(ab+bc+ac)`
`A=(ab+bc+ac)/3`
`text{Using given relationship, where}\ x_1=ab, x_2=bc, …`
| `(ab xx bc xx ca)/((ab+bc+ac)/3)^3` | `<=1` | |
| `(ab xx bc xx ca)` | `<=((ab+bc+ac)/3)^3` | |
| `(abc)^2` | `<=((2(ab+bc+ac))/6)^3` | |
| `abc` | `<=(S/6)^(3/2)` |
ii. `text{If prism is a cube}\ \ a=b=c`
`=> V=a^3, \ \ S=6a^2`
`(S/6)^(3/2)=((6a^2)/6)^(3/2)=a^3`
`text{In the case of a cube:}`
`V=(S/6)^(3/2)`
`text{Also,}\ \ V<=(S/6)^(3/2)\ \ \ text{(using part (i))}`
`:.\ text{For rectangular prisms with a given surface area, a cube}`
`text{has the maximum volume.}`
A square in the Argand plane has vertices
`5+5i,quad5-5i,quad-5-5i` and `-5+5i`.
The complex numbers `z_A=5+i, z_B` and `z_C` lie on the square and form the vertices of an equilateral triangle, as shown in the diagram.
Find the exact value of the complex number `z_B`. (4 marks)
`(5-16/sqrt3)+5i`
`z_A=5+i, \ \ z_B=b+5i, \ \ z_C=c-5i`
`z_B-z_A=(b-5)+4i`
`z_C-z_A=(c-5)-6i`
`text{Internal angles of equilateral triangle} = pi/3:`
`=>\ (z_B-z_A)\ text{is an anti-clockwise rotation of}\ (z_C-z_A)\ text{by}\ pi/3`
`e^(i pi/3)(z_B-z_A)=z_C-z_A`
`(1/2+i sqrt3/2)((b-5)+4i)=(c-5)-6i`
| `(b-5)/2+2i+((b-5)sqrt3)/2 i-2sqrt3` | `=(c-5)-6i` | |
| `(b-5-4sqrt3)/2 + i((4+(b-5)sqrt3)/2)` | `=(c-5)-6i` |
`text{Equating imaginary parts:}`
| `(4+(b-5)sqrt3)/2` | `=-6` | |
| `(b-5)sqrt3` | `=-16` | |
| `b-5` | `=-16/sqrt3` | |
| `b` | `=5-16/sqrt3` |
`:.z_B=(5-16/sqrt3)+5i`
Members of steel trusses are often connected using metal rivets as shown.
Describe the hot working process that the rivet has undergone to create the resulting structure. Support your answer by completing and labelling the diagram of the sectioned rivet. (3 marks)
--- 7 WORK AREA LINES (style=lined) ---
Use the following diagram to answer Questions 19-20.
The diagram shows how CRISPR/Cas9 can be used as a new tool for genetic engineering. This technology has dramatically improved scientists' ability to successfully modify genomes.
Question 19
What type of structure must Cas9 be?
Question 20
Scientists have been able to use biotechnology to 'cut and paste' DNA for decades.
Why would the new CRISPR/Cas9 technology have improved the scientists' success in cutting DNA of specific genes?
Question 19: `A`
Question 20: `D`
Question 19
`=>A`
Question 20
`=>D`
The pedigree shows the inheritance of a genetic disorder.
Which row of the table correctly identifies the two possible types of inheritance for this disorder?
\begin{align*}
\begin{array}{l}
\rule{0pt}{2.5ex} \ \rule[-1ex]{0pt}{0pt}& \\
\ \rule[-1ex]{0pt}{0pt}& \\
\rule{0pt}{2.5ex}\textbf{A.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{B.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{C.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{D.}\rule[-1ex]{0pt}{0pt}\\
\end{array}
\begin{array}{|c|c|c|c|}
\hline
\rule{0pt}{2.5ex}\quad \textit{Autosomal} \quad &\quad \textit{Autosomal} \quad & \quad \textit{Sex-linked} \quad & \quad \textit{Sex-linked}\quad \\
\textit{dominant} &\textit{recessive} \rule[-1ex]{0pt}{0pt}& \textit{dominant} & \textit{recessive}\\
\hline
\rule{0pt}{2.5ex}\checkmark \rule[-1ex]{0pt}{0pt}& & \checkmark & \\
\hline
\rule{0pt}{2.5ex}\checkmark \rule[-1ex]{0pt}{0pt}& & & \checkmark \\
\hline
\rule{0pt}{2.5ex}& \checkmark \rule[-1ex]{0pt}{0pt}& \checkmark & \\
\hline
\rule{0pt}{2.5ex}& \checkmark \rule[-1ex]{0pt}{0pt}& & \checkmark \\
\hline
\end{array}
\end{align*}
\(A\)
By Elimination:
\(\Rightarrow A\)
Compound `text{X}` shows three signals in its \(\ce{^{13}C NMR}\) spectrum.
Treatment of `text{X}` with hot acidified potassium permanganate produces a compound `text{Y}`. Compound `text{Y}` turns blue litmus red.
Compound `text{X}` produces compound `text{Z}` upon reaction with hot concentrated sulfuric acid.
Which of the following correctly identifies compounds `text{X}`, `text{Y}` and `text{Z}`?
`D`
`=>D`
Consider the following equilibrium.
\(\ce{HF(aq) + CF3COO-(aq) \rightleftharpoons F-(aq) + CF3COOH(aq) \ \ \ \ \ \ $K_{eq}$ = 3.80 \times 10^{-4}} \)
Which row of the table correctly identifies the strongest acid and the strongest base in this system?
| \(\text{Strongest acid}\) | \(\text{Strongest base}\) | |
| A. | \(\ce{CF3OOH(aq)}\) | \(\ce{F-(aq)}\) |
| B. | \(\ce{CF3OOH(aq)}\) | \(\ce{CF3OO-(aq)}\) |
| C. | \(\ce{HF(aq)}\) | \(\ce{F-(aq)}\) |
| D. | \(\ce{HF(aq)}\) | \(\ce{CF3OO-(aq)}\) |
`A`
`=>A`
Researchers have identified a gene that determines the inflammatory response of lung cells to infection with a virus. An allele of this gene is associated with increased inflammation and increased chance of death from the virus.
The table shows the percentage presence of the allele in people with different ancestries.
Explain how mutation, natural selection, genetic drift and gene flow could have led to these differences in the gene pools of populations with differing ancestry. (7 marks)
--- 15 WORK AREA LINES (style=lined) ---
Mutation
Natural selection
Gene flow
Genetic drift
Mutation
Natural selection
Gene flow
Genetic drift
Outline the ways in which the DNA of prokaryotes and eukaryotes differ. (3 marks)
--- 6 WORK AREA LINES (style=lined) ---
Renal dialysis involves passing blood from a patient past a dialysate solution in order to remove waste such as urea from the blood.
Which diagram correctly shows possible concentrations of urea and the direction of flow of both solutions in a dialysis machine?
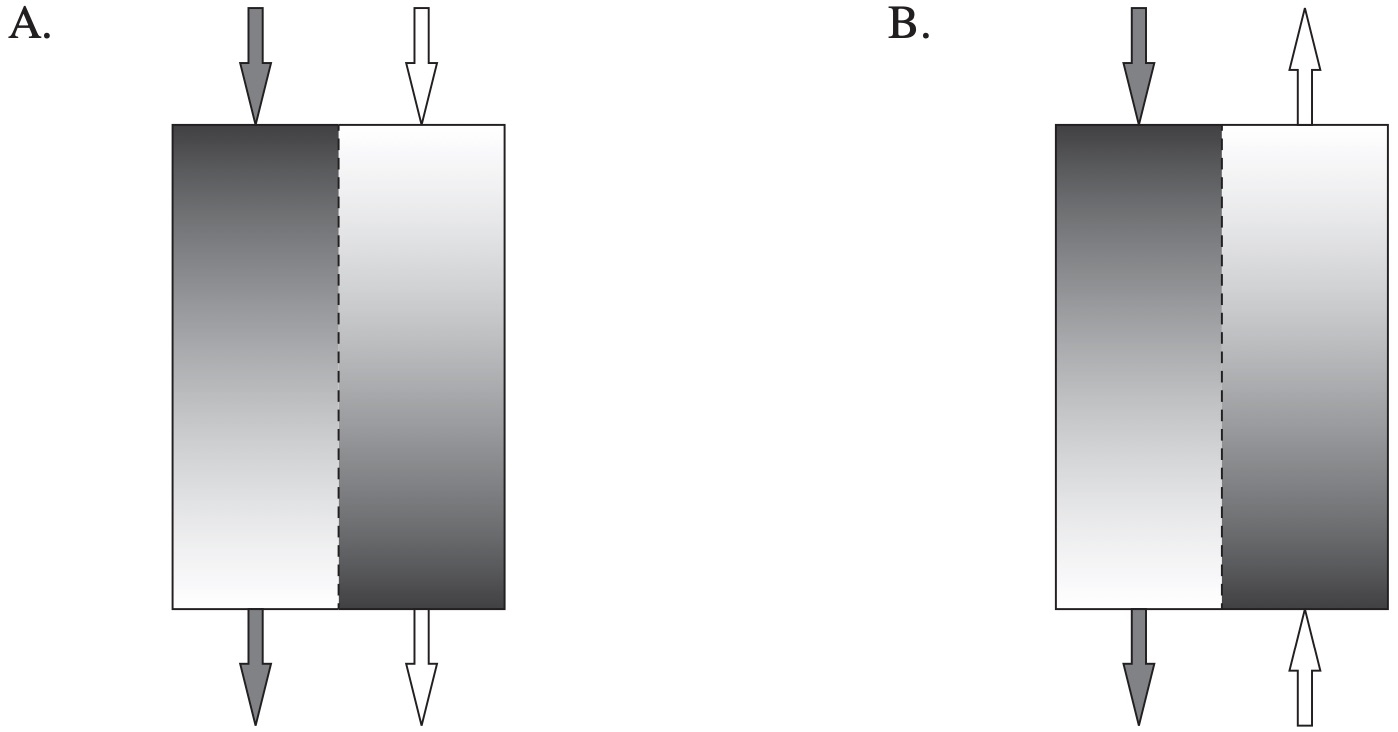
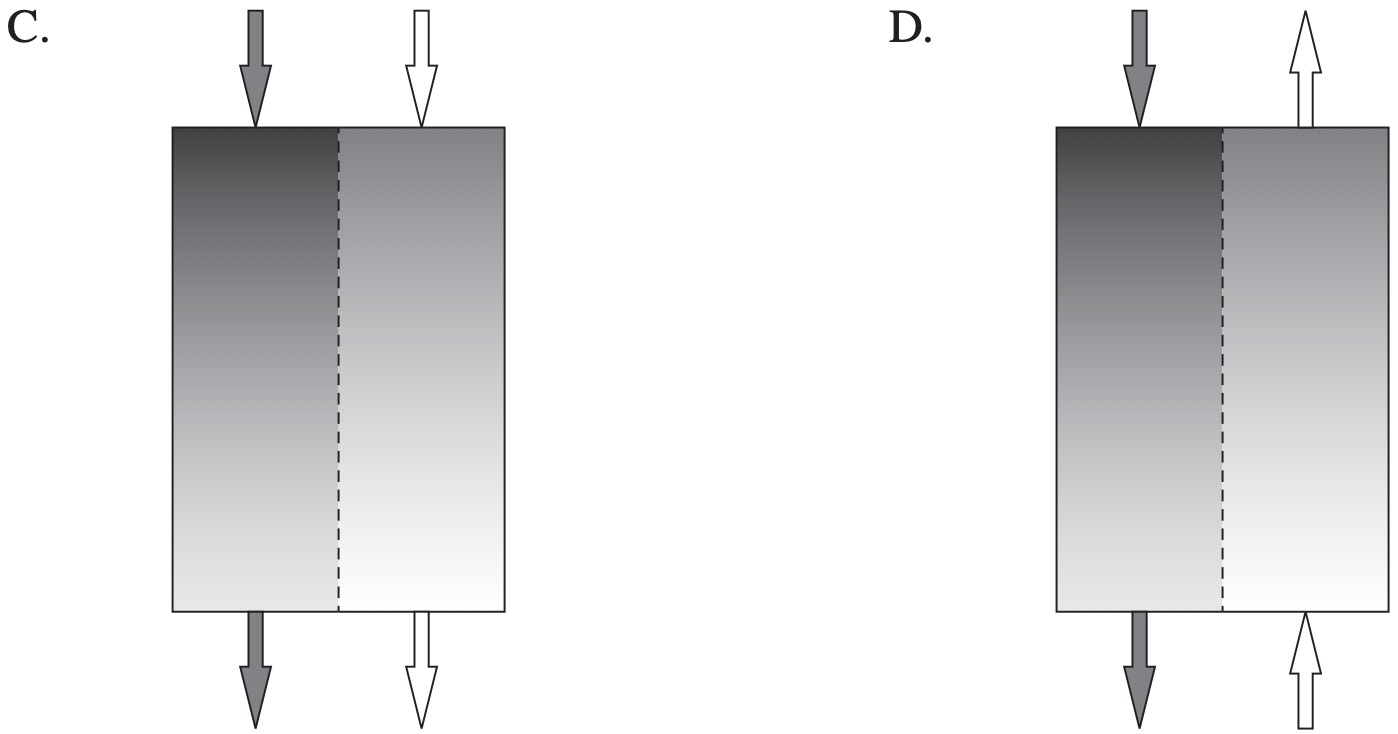
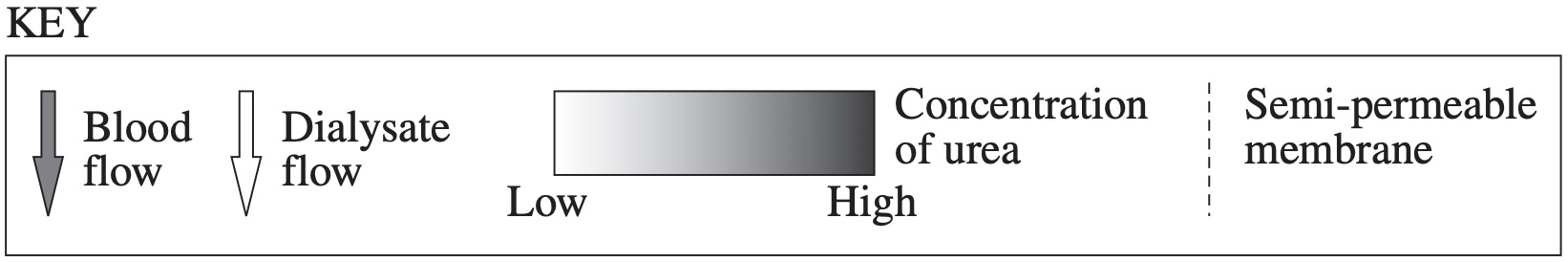
`D`
By Elimination:
`=>D`
A cured cylindrical concrete specimen is tested under a compressive load through its vertical axis, until it fails.
Which of the following is the most likely shape of the front view of the failed specimen?
`A`
`=>A`
Which electronic component can be used to increase or decrease the speed of a train powered by an electric motor?
`D`
`=>D`
--- 3 WORK AREA LINES (style=lined) ---
--- 5 WORK AREA LINES (style=lined) ---
The diagram below shows the tetrahedron with vertices `A, B, C` and `S`.
The point `K` is defined by `vec(SK)=(1)/(4) vec(SB)+(1)/(3) vec(SC)`, as shown in the diagram.
The point `L` is the point of intersection of the straight lines `S K` and `B C`.
--- 6 WORK AREA LINES (style=lined) ---
--- 5 WORK AREA LINES (style=lined) ---
| i. | `lambda vecu+mu vecv` | `=0` |
| `lambda vecu` | `=-mu vecv` |
`lambda=0\ \ text{or}\ \ vecu=-(mu/lambda)vecv=k vecv\ \ (kinRR)`
`text{S}text{ince}\ \ vecu and vecv\ \ text{are not parallel}`
`=> lambda=mu=0`
ii. `\lambda_1 \vec{u}+\mu_1 \vec{v}=\lambda_2 \vec{u}+\mu_2 \vec{v}`
| `\lambda_1 \vec{u}-\lambda_2 \vec{u}+\mu_1 \vec{v}-\mu_2 \vec{v}` | `=vec0` | |
| `(\lambda_1-\lambda_2)\vec{u}+(\mu_1-\mu_2)\vec{v}` | `=vec0` |
`text{Using part (i):}`
`(\lambda_1-\lambda_2)=0 and (\mu_1-\mu_2)=0`
`:.\lambda_1=\lambda_2 and \mu_1=\mu_2\ …\ text{as required}`
| iii. | `vec(BL)` | `=lambda vec(BC)` |
| `=lambda(vec(BS)+vec(SC))\ \ \ …\ (1)` |
| `vec(BL)` | `=vec(BS)+mu vec(SK)` | |
| `=-vec(SB)+mu(1/4vec(SB)+1/3vec(SC))` | ||
| `=-vec(SB)+mu/4vec(SB)+mu/3vec(SC)` | ||
| `=mu/3vec(SC)+(mu/4-1)vec(SB)\ \ \ …\ (2)` |
`text{Using}\ \ (1) = (2):`
`lambda vec(BS)+ lambda vec(SC)=mu/3vec(SC)+(mu/4-1)vec(SB)`
`mu/3=lambda, \ \ 1-mu/4=lambda`
| `mu/3` | `=1-mu/4` | |
| `(4mu+3mu)/12` | `=1` | |
| `(7mu)/12` | `=1` | |
| `mu` | `=12/7` |
`lambda=(12/7)/3=4/7`
`:.vec(BL)=(4)/(7) vec(BC)\ \ text{… as required}`
iv. `vec(AP)=-6 vec(AB)-8 vec(AC)`
`text{If}\ P\ text{lies on}\ AL, ∃k\ text{such that}\ \ vec(AP)=k vec(AL)`
| `-6 vec(AB)-8 vec(AC)` | `=k vec(AL)` | |
| `-6 vec(AB)-8 (vec(AB)+vec(BC))` | `=k(vec(AB)+vec(BL))` | |
| `-14 vec(AB)-8vec(BC)` | `=k(vec(AB)+4/7vec(BC))\ \ text{(see part (iii))}` | |
| `-14 vec(AB)-8vec(BC)` | `=kvec(AB)+(4k)/7vec(BC)` |
`k=-14, \ \ (4k)/7=-8\ \ =>\ \ k=-14`
`:.\ P\ text{lies on}\ \ AL.`
A truss is fixed to a wall at `A` and `B` as shown. Ignore the mass of the truss.
--- 4 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
i. `3.75\ text{kN ←}`
ii. `AC=4802\ text{N}`
iii. `CE = 3\ text{kN (tension)}`
i. Horizontal reaction at `A`
| \(\circlearrowright \Sigma \text{M}_\text{B}\) | \(= 0\) | |
| `0` | `= -(A_H xx 4\ text{m}) + (1500\ text{N} xx 10\ text{m})` | |
| `A_H` | `= 3750\ text{N}= 3.75\ text{kN ←}` |
Rabies is a disease that can affect all mammals and is caused by the rabies virus. It is transmitted by the bite of an infected animal. Without treatment it almost always results in death.
The rabies virus is a single-stranded RNA virus. It contains and codes for only five proteins. The diagrams show the structure and reproduction of the virus.
Post exposure prophylaxis (PEP) is given to patients who have been bitten by a rabid animal.
PEP includes an injection of human rabies antibodies (HRIG) as well as injections of a rabies vaccine at 0, 3, 7 and 14 days after exposure to the virus.
The following graphs show a generalised response to rabies infection without and with PEP.
Explain how PEP prevents rabies developing after infection with the virus. Support your answer with reference to the information and data provided above. (8 marks)
--- 20 WORK AREA LINES (style=lined) ---
Once the rabies virus has entered the wound:
The rabies vaccine works by:
Once the rabies virus has entered the wound:
The rabies vaccine works by:
--- 12 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
a. Glucose Levels
Insulin Levels
Glucagon Levels
b. Differences in temperature vs glucose maintenance
a. Glucose Levels
Insulin Levels
Glucagon Levels
b. Differences in temperature vs glucose maintenance
--- 6 WORK AREA LINES (style=lined) ---
--- 6 WORK AREA LINES (style=lined) ---
b. The Effect of Meiosis on Genetic Variation
Independent Assortment
Random Segregation
Crossing Over
b. The Effect of Meiosis on Genetic Variation
Independent Assortment
Random Segregation
Crossing Over
Excess solid calcium hydroxide is added to a beaker containing 0.100 L of 2.00 mol L¯1 hydrochloric acid and the mixture is allowed to come to equilibrium.
--- 4 WORK AREA LINES (style=lined) ---
--- 10 WORK AREA LINES (style=lined) ---
a. \(\ce{Ca(OH)2 (s) + 2 HCl (aq) -> CaCl2 (aq) + 2 H2O (l)}\)
`text{n(HCl)} = text{c} xx text{V} = 2.00 xx 0.100 = 0.200\ text{mol}`
\[\ce{n(Ca(OH)2) = \frac{n(HCL)}{2} = \frac{0.200}{2}= 0.100 mol}\]
b. `text{pH} = 11.35`
a. \(\ce{Ca(OH)2 (s) + 2 HCl (aq) -> CaCl2 (aq) + 2 H2O (l)}\)
`text{n(HCl)} = text{c} xx text{V} = 2.00 xx 0.100 = 0.200\ text{mol}`
\[\ce{n(Ca(OH)2) = \frac{n(HCL)}{2} = \frac{0.200}{2}= 0.100 mol}\]
b. \(\ce{Ca(OH)2(s) \rightleftharpoons Ca^2+ (aq) + 2 OH– (aq)}\)
`[text{Ca}^(2+)] = text{n} / text{V} = 0.100 / 0.100 = 1.00\ text{mol L}^-1`
| \(\ce{K_{sp}}\) | \( \ce{= [Ca^2+][OH– ]^2}\) | |
| `5.02 xx 10^(-6)` | `= 1.00 xx [text{OH}^– ]^2` | |
| `[text{OH}^– ]` | `=sqrt{5.02 xx 10^(-6)}=2.24 xx 10^(−3)\ text{mol L}^(-1)` | |
| `text{pOH }` | `= −log_10(2.24 xx 10^(-3))= 2.650` |
`:.\ text{pH} = 14-2.650 = 11.35`
An experiment is set up as shown.
When the switch is closed, the reading on the spring balance changes immediately, then returns to the initial reading.
Which row of the table correctly shows the direction of the current through the straight conductor \(XY\) and the direction in which the pointer on the spring balance initially moves?
\begin{align*}
\begin{array}{l}
\textit{}& \textit{} \\
\textit{}& \textit{} \\
\rule{0pt}{2.5ex}\textbf{A.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{B.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{C.}\rule[-1ex]{0pt}{0pt}\\
\rule{0pt}{2.5ex}\textbf{D.}\rule[-1ex]{0pt}{0pt}\\
\end{array}
\begin{array}{|c|c|}
\hline
\textit{Direction of current through}& \textit{Direction in which the} \\
\textit{the straight conductor}& \textit{pointer initially moves} \\
\hline
\rule{0pt}{2.5ex}\text{From \(X\) to \(Y\)}\rule[-1ex]{0pt}{0pt}&\text{Down}\\
\hline
\rule{0pt}{2.5ex}\text{From \(X\) to \(Y\)}\rule[-1ex]{0pt}{0pt}& \text{Up}\\
\hline
\rule{0pt}{2.5ex}\text{From \(Y\) to \(X\)}\rule[-1ex]{0pt}{0pt}& \text{Down} \\
\hline
\rule{0pt}{2.5ex}\text{From \(Y\) to \(X\)}\rule[-1ex]{0pt}{0pt}& \text{Up} \\
\hline
\end{array}
\end{align*}
\(B\)
\(\Rightarrow B\)
When a train is at rest in a tunnel, the train is slightly longer than the tunnel.
In a thought experiment, the train is travelling from left to right fast enough relative to the tunnel that its length contracts and it fits inside the tunnel.
An observer on the ground sets up two cameras, at `X` and `Y`, to take photos at exactly the same time. The photos show that both ends of the train are inside the tunnel.
A passenger travelling on the train at its centre can see both ends of the tunnel and is later shown the photos.
From the point of view of the passenger, what is observed and what can be deduced about the photos?
`A`
`=>A`
A drive mechanism used to control the satellite dish of a mobile TV transmission van is shown.
Key dimensions of components are given.
The dimensions of each of the five holes in the gear are \( \phi 10 \vee \phi 16 \times 90°\) on one side.
A partially sectioned drawing of the gear and shaft is provided below.
Complete the sectioned assembly of this drive mechanism to AS 1100 from the direction indicated by the arrow. Include break lines where appropriate. (6 marks)
--- 0 WORK AREA LINES (style=lined) ---
Critical aspects of this drawing:
Nitrogen dioxide reacts to form dinitrogen tetroxide in a sealed flask according to the following equation.
`2 text{NO}_(2)(g) ⇌ text{N}_(2) text{O}_(4)(g) qquadqquad Delta H =-57.2 \ text{kJ mol}^(-1)`
Which graph best represents the rates of both the forward and reverse reactions when an equilibrium system containing these gases is cooled at time `t` ?
`D`
`=> D`
An aqueous solution of sodium hydrogen carbonate has a pH greater than 7 .
Which statement best explains this observation?
`A`
`=> A`
Analyse how a student could design a chemical synthesis process to be undertaken in the school laboratory. In your response, use a specific process relating to the synthesis of an organic compound, including a chemical equation, and refer to:
--- 25 WORK AREA LINES (style=lined) ---
Selecting reagents
Acetic acid + ethanol ⇌ Ethyl ethanoate + water
Reaction conditions
Potential hazards and safety precautions
Yield and purity
Selecting reagents
Acetic acid + ethanol ⇌ Ethyl ethanoate + water
Reaction conditions
Potential hazards and safety precautions
Yield and purity
The concentration of citric acid, a triprotic acid, in a carbonated soft drink was to be determined.
Step 1: A solution of \( \ce{NaOH(aq)} \) was standardised by titrating it against 25.00 mL aliquots of a solution of the monoprotic acid potassium hydrogen phthalate \( \ce{(KHP)} \). The \( \ce{(KHP)} \) solution was produced by dissolving 4.989 g in enough water to make 100.0 mL of solution. The molar mass of \( \ce{(KHP)} \) is 204.22 g mol ¯1.
The results of the standardisation titration are given in the table.
Step 2: A 75.00 mL bottle of the drink was opened and the contents quantitatively transferred to a beaker. The soft drink was gently heated to remove \( \ce{CO2}\).
Step 3: The cooled drink was quantitatively transferred to a 250.0 mL volumetric flask and distilled water was added up to the mark.
Step 4: 25.00 mL samples of the solution were titrated with the \( \ce{NaOH(aq)}\) solution. The average volume of \( \ce{NaOH(aq)} \) used was 13.10 mL.
--- 20 WORK AREA LINES (style=lined) ---
--- 5 WORK AREA LINES (style=lined) ---
a. `0.1298 text{mol L}^(–1)`
b. \( \ce{CO2} \) can dissolve in water to produce \( \ce{H2CO3}\):
\( \ce{CO2(g) + H2O(l) \rightleftharpoons H2CO3(aq)} \)
a. \( \ce{KHP(aq) + NaOH(aq) -> NaKP(aq) + H2O(l)} \)
`text{n(HX)}= 4.989 / 204.22= 0.02443\ text{mol}`
`[text(HX)]= \text{n}/\text{V}= 0.02443 / 0.1000= 0.2443 text{mol L}^(–1)`
`text{n(HX) titrated} = text{c} xx text{V}= 0.2443 xx 0.02500= 0.0006107\ text{mol}`
`=>\ text{n(NaOH)}= 0.0006107 text{mol}`
Eliminate the first trial because it is an outlier.
`text{V}_(text(avg))text{(NaOH)}= 1 / 3 xx (27.40 + 27.20 + 27.60)= 27.40\ text{mL}`
`text{[NaOH]}= text{n} / text{V}= [6.107 xx 10^−3] / 0.02740 = 0.2229 text{mol L}^(–1)`
\( \ce{H3X(aq) + 3NaOH(aq) -> Na3X(aq) + 3H2O(l)} \)
`text{n(NaOH) titrated}= text{c} xx text{V}=0.2229 xx 0.01310= 2.920 xx 10^(−3)\ text{mol}`
`text{n(H}_3 text{X)}= 1/3 xx 2.920 xx 10^(−3)= 9.733 xx 10^(−4)\ text{mol}`
`text{[H}_3 text{X] diluted} = text{n} / text{V} = (9.733 xx 10^(−4) )/ 0.025= 0.03893 \ text{mol L}^(–1)`
`text{[H}_3 text{X] original}= 250.0 / 75.00 xx 0.03893= 0.1298 text{mol L}^(–1)`
Therefore, the concentration of citric acid in the soft drink is 0.1298 mol L¯1.
b. \( \ce{CO2} \) can dissolve in water to produce \( \ce{H2CO3}\):
\( \ce{CO2(g) + H2O(l) \rightleftharpoons H2CO3(aq)} \)
This would enable \( \ce{NaOH} \) to react with \( \ce{H2CO3:} \)
\( \ce{2NaOH(aq) + H2CO3(aq) -> Na2CO3(aq) + 2H2O(l)} \)
A low molecular weight biopolymer is being investigated for its suitability for medical use. In one trial a molecular weight of `2900 pm 100\ text{g}\ text{mol}^(-1)` proved to be optimum.
A section of this biopolymer is shown.
Which will produce the suitable biopolymer?
`D`
This polymer is a condensation polymer, meaning that it is formed through the reaction between monomers that consist of a carboxylic acid and/or an alcohol functional group, with the elimination of water.
Thus, if `text{n}` monomers react to form this polyester, `text{(n – 1)}` molecules of water would be eliminated.
Calculating their molar masses:
| `text{n}\ xx\ text{monomers}` | `= text{polymer} + text{(n – 1)} xx text{H}_2 text{O}` |
| `text{n} xx (90.078)` | `= 2900 + text{(n – 1)} xx (18.016)` |
| `text{n}(90.078-18.016)` | `=2900-18.016` |
| `:.\ text{n}` | `=2881.984/72.062=39.99…` |
`=> D`
A 2.0 g sample of silver carbonate (MM = 275.81 g mol ¯1) was added to 100.0 mL of water in a beaker. The solubility of silver carbonate at this temperature is `1.2 × 10^(-4)` mol L ¯1. It was then diluted by adding another 100.0 mL of water.
What is the ratio of the concentration of silver ions in solution before and after dilution?
`A`
The maximum moles of `text{Ag}_2 text{CO}_3` that can be dissolved in 100.0 mL is:
| `text{n(Ag}_2 text{CO}_3 text{)}_max` | `= 1.2 × 10^(−4) xx 0.1000` |
| `= 1.2 xx 10^(−5) text{mol}` |
The number of moles of silver carbonate added to the water is:
| `text{n(Ag}_2 text{CO}_3)` | `= text{m} / text{MM}` |
| `= 2.0 / 275.81` | |
| `= 7.2514 xx 10 ^(-3) text{mol}` |
`=> A`
In ten years, the future value of an investment will be $150 000. The interest rate is 4% per annum, compounded half-yearly.
Which equation will give the present value `(P V)` of the investment?
`D`
`text{Compounding periods} = 10 xx 2 = 20`
`text{Compounding rate} = (4text{%})/2 = 2text{%} = 0.02`
`PV=(150\ 000)/((1+0.02)^(20))`
`=>D`
A manufacturer requires that its product contains at least 85% v/v ethanol.
The concentration of ethanol in water can be determined by a back titration. Ethanol is first oxidised to ethanoic acid using an excess of acidified potassium dichromate solution.
\(\ce{3C2H5OH($aq$) + 2Cr2O7^2-($aq$) + 16H^+($aq$) ->3CH3COOH($aq$) + 4Cr^3+($aq$) + 11H2O($l$)}\)
The remaining dichromate ions are reacted with excess iodide ions to produce iodine \(\ce{(I2)}\)
\(\ce{Cr2O7^2-($aq$) + 14H^+($aq$) + 61^-($aq$) -> 2Cr^3+($aq$) + 7H2O($l$) + 3I2($aq$)}\)
The iodine produced is then titrated with sodium thiosulfate \(\ce{(Na2S2O3)}\).
\(\ce{I2($aq$) + 2S2O3^2-($aq$) -> 2I^-($aq$) + S4O6^2-($aq$)}\)
A 25.0 mL sample of the manufacturer's product was diluted with distilled water to 1.00 L. A 25.0 mL aliquot of the diluted solution was added to 20.0 mL of 0.500 mol L¯1 acidified potassium dichromate solution in a conical flask. Potassium iodide (5.0 g) was added and the solution titrated with 0.900 mol L¯1 sodium thiosulfate. This was repeated three times.
The following results were obtained.
The density of ethanol is 0.789 g mL¯1.
Does the sample meet the manufacturer's requirements? Support your answer with calculations. (7 marks)
--- 22 WORK AREA LINES (style=lined) ---
| \( \text{V}_\text{avg}\ (\ce{Na2S2O3}) \) | `= (28.7 + 28.4 + 28.6) / 3` |
| `= 28.5666… text{mL}` | |
| `= 0.0285666… text{L}` | |
`text{n(Na}_2 text{S}_2 text{O}_3text{)}=\ text{c} xx text{V}= 0.900 xx 0.0285666…= 0.02571 text{mol}`
\( \ce{n(S2O3^2-) = n(Na2S2O3) = 0.02571\ \text{mol}} \)
`text{I}_2 text{and S}_2 text{O}_3 ^(2-)\ text{are in a}\ 1 : 2\ text{ratio:}`
`text{n(I}_2text{)} = 1/2 xx text{n(S}_2 text{O}_3^( 2-) text{)}= 1/2 xx 0.02571= 0.012855\ text{mol}`
`text{Excess Cr}_2 text{O}_7^(\ \ 2-) text{and I}_2 text{are in}\ 1:3\ text{ratio:}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) excess}= 1/3 xx text{n(I}_2 text{)}= 1/3 xx 0.012855= 0.004285\ text{mol}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) initial}= text{c} xx text{V}= 0.500 xx 20/1000= 0.01\ text{mol}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) reacted with ethanol}`
`= text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) initial}-text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) excess}`
`= 0.01-0.004285`
`= 0.005715\ text{mol}`
`text{n(C}_2 text{H}_5 text{OH)}= 3/2 xx text{n(Cr}_2 text{O}_7^(\ \ 2-) text{)}= 3/2 xx 0.005715= 0.0085725\ text{mol}`
`text{m(C}_2 text{H}_5 text{OH)}= text{n} xx text{MM}=0.0085725 xx (2 xx 12.01 + 6 xx 1.008 + 16.00)= 0.3949\ text{g}`
Find the mass of ethanol in the original solution:
`text{m(C}_2 text{H}_5 text{OH) original}= 0.3949… xx 1000/25= 15.796…\ text{g}`
`text{D}= text{m} / text{V}\ \ =>\ \ text{V}= text{m} / text{D}`
`text{V(C}_2 text{H}_5 text{OH)}= 15.796 / 0.789= 20.021\ text{mL}`
`text{% (C}_2text{H}_5text{OH)}= text{V(ethanol)} / text{V(sample)}= 20.021 / 25.0= 80.08… %\ text{v}//text{v}`
| \( \text{V}_\text{avg}\ (\ce{Na2S2O3}) \) | `= (28.7 + 28.4 + 28.6) / 3` |
| `= 28.5666… text{mL}` | |
| `= 0.0285666… text{L}` | |
`text{n(Na}_2 text{S}_2 text{O}_3text{)}=\ text{c} xx text{V}= 0.900 xx 0.0285666…= 0.02571 text{mol}`
\( \ce{n(S2O3^2-) = n(Na2S2O3) = 0.02571\ \text{mol}} \)
`text{I}_2 text{and S}_2 text{O}_3 ^(2-)\ text{are in a}\ 1 : 2\ text{ratio:}`
`text{n(I}_2text{)} = 1/2 xx text{n(S}_2 text{O}_3^( 2-) text{)}= 1/2 xx 0.02571= 0.012855\ text{mol}`
`text{Excess Cr}_2 text{O}_7^(\ \ 2-) text{and I}_2 text{are in}\ 1:3\ text{ratio:}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) excess}= 1/3 xx text{n(I}_2 text{)}= 1/3 xx 0.012855= 0.004285\ text{mol}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) initial}= text{c} xx text{V}= 0.500 xx 20/1000= 0.01\ text{mol}`
`text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) reacted with ethanol}`
`= text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) initial}-text{n(Cr}_2 text{O}_7^(\ \ 2-) text{) excess}`
`= 0.01-0.004285`
`= 0.005715\ text{mol}`
`text{n(C}_2 text{H}_5 text{OH)}= 3/2 xx text{n(Cr}_2 text{O}_7^(\ \ 2-) text{)}= 3/2 xx 0.005715= 0.0085725\ text{mol}`
`text{m(C}_2 text{H}_5 text{OH)}= text{n} xx text{MM}=0.0085725 xx (2 xx 12.01 + 6 xx 1.008 + 16.00)= 0.3949\ text{g}`
Find the mass of ethanol in the original solution:
`text{m(C}_2 text{H}_5 text{OH) original}= 0.3949… xx 1000/25= 15.796…\ text{g}`
`text{D}= text{m} / text{V}\ \ =>\ \ text{V}= text{m} / text{D}`
`text{V(C}_2 text{H}_5 text{OH)}= 15.796 / 0.789= 20.021\ text{mL}`
`text{% (C}_2text{H}_5text{OH)}= text{V(ethanol)} / text{V(sample)}= 20.021 / 25.0= 80.08… %\ text{v}//text{v}`
The molar enthalpies of neutralisation of three reactions are given.
Reaction 1:
\(\ce{HCl($aq$) + KOH($aq$) -> KCl($aq$) + H2O($l$)}\) \(\ce{Δ$H$}\) \(\pu{=-57.6 kJ mol-1}\)
Reaction 2:
\(\ce{HNO3($aq$) + KOH($aq$) -> KNO3($aq$) + H2O($l$)}\) \(\ce{Δ$H$}\) \(\pu{=-57.6 kJ mol-1}\)
Reaction 3:
\(\ce{HCN($aq$) + KOH($aq$) -> KCN($aq$) + H2O($l$)}\) \(\ce{Δ$H$}\) \(\pu{=-12.0 kJ mol-1}\)
Explain why the first two reactions have the same enthalpy value but the third reaction has a different value. (4 marks)
--- 10 WORK AREA LINES (style=lined) ---
The diagram shows a simply supported beam in equilibrium. It is loaded with a single force (`text{F}`) as shown.
Which of the following angles is closest to the angle of the reaction force to the horizontal at the fixed bearing?
`B`
`=>B`