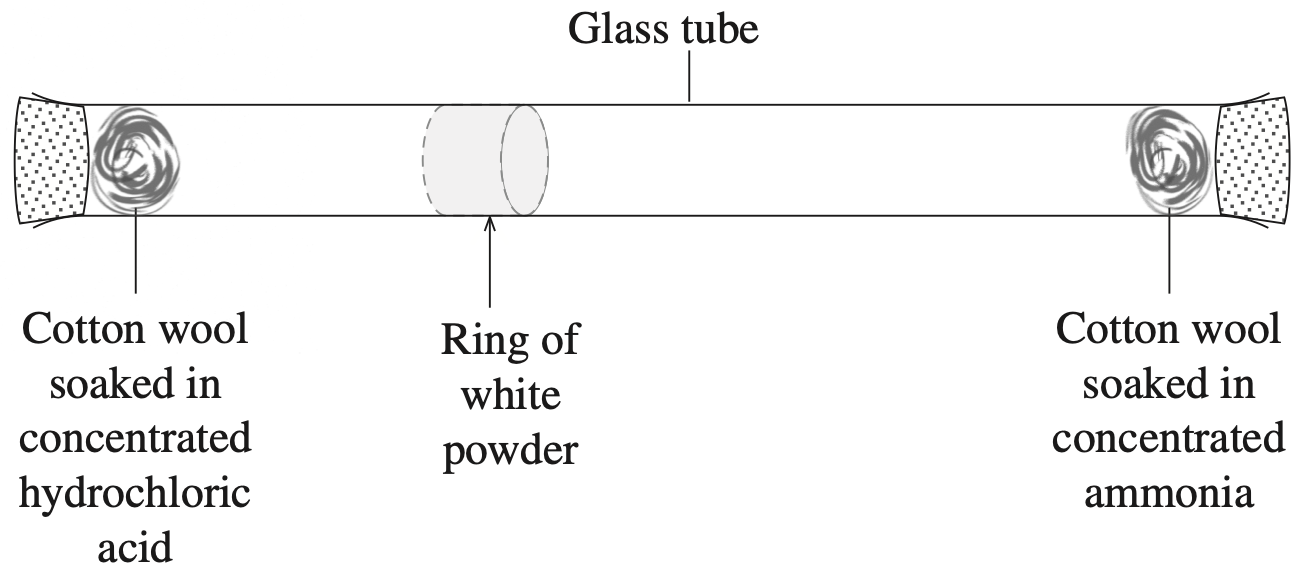
Refer to the following information to answer Questions 19 and 20.
Question 19
What can be inferred from the scientists' discovery?
- Cancer cells carry unique antigens.
- Self-antigens are not present on cancer cells.
- The melanoma patient has a dysfunctional immune system.
- The body cannot mount an immune response against cancer cells.
Question 20
The effect of the melanoma vaccine is to stimulate
- T cells which produce antibodies.
- cytotoxic T cells which activate B cells.
- cell division to produce more lymphocytes.
- production of B cells which destroy melanoma cells.